Korean Circ J.
2013 Jul;43(7):481-487. 10.4070/kcj.2013.43.7.481.
Cardiac Rehabilitation Increases Exercise Capacity with a Reduction of Oxidative Stress
- Affiliations
-
- 1Health Center, Hitotsubashi University, Tokyo, Japan. t.fukuda@r.hit-u.ac.jp
- 2Department of Ischemic Circulatory Physiology, University of Tokyo, Tokyo, Japan.
- 3Department of Cardiovascular Medicine, University of Tokyo, Tokyo, Japan.
- KMID: 2145504
- DOI: http://doi.org/10.4070/kcj.2013.43.7.481
Abstract
- BACKGROUND AND OBJECTIVES
Reactive oxygen species (ROS) mediate various signaling pathways that underlie vascular inflammation in atherogenesis and cardiovascular diseases. Cardiac rehabilitation (CR) has a variety of multiple beneficial effects, including anti-inflammatory effects. The purpose of the present study was to investigate the effects of CR on ROS in patients with cardiovascular diseases.
SUBJECTS AND METHODS
The serum level of derivatives of reactive oxidative metabolites, an index of oxidative stress, was measured in 100 patients with cardiovascular diseases before, and, subsequently, 3 and 6 months after, CR. A biological antioxidant potential (BAP) test was applied to assess the antioxidant power of the serum.
RESULTS
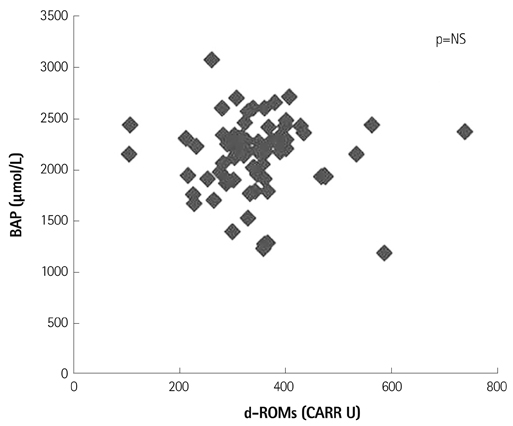
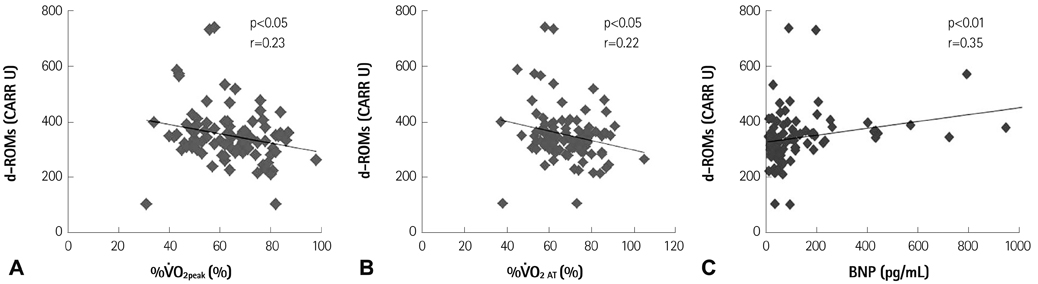
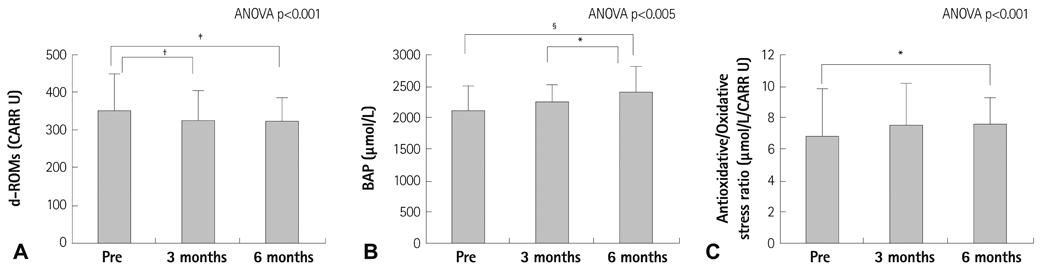
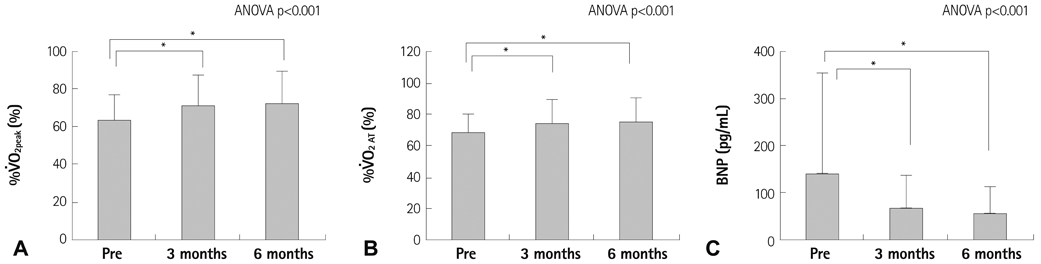
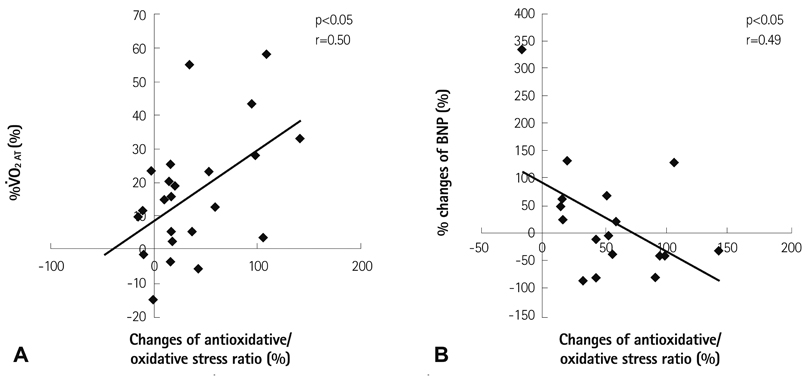
The resting reactive oxidative metabolite levels decreased 3-6 months after CR {pre: 351+/-97 Carratelli unit (CARR U), 3 months: 329+/-77 CARR U, 6 months: 325+/-63 CARR U, all p<0.01} with the increase of the percentage of the predicted values of VO2 peak and the percentage of the predicted values of VO2 at the anaerobic threshold (VO2 AT) and the decrease of the B-type natriuretic peptide (BNP). The BAP test and antioxidative/oxidative stress ratio increased 6 months after CR. The % changes of the antioxidative/oxidative stress ratio was positively correlated with the % changes of VO2 AT, and negatively correlated with the % changes of the BNP.
CONCLUSION
These results suggest that intensive supervised CR significantly improved exercise capacity, which may be attributable to an adaptive response involving more efficient oxidative metabolites or the increased capacity of endogenous anti-oxidative systems in patients with cardiovascular diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Tousoulis D, Briasoulis A, Papageorgiou N, et al. Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov. 2011; 6:103–114.2. Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003; 91:7A–11A.3. Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007; 93:903–907.4. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005; 115:500–508.5. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113:1708–1714.6. Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005; 111:555–562.7. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247.8. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011; 301:H2181–H2190.9. Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993; 328:538–545.10. Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002; 22:1869–1876.11. LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005; 99:1205–1213.12. Judge S, Jang YM, Smith A, et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R1564–R1572.13. Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005; 111:1763–1770.14. Cesarone MR, Belcaro G, Carratelli M, et al. A simple test to monitor oxidative stress. Int Angiol. 1999; 18:127–130.15. Gerardi G, Usberti M, Martini G, et al. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002; 40:104–110.16. Lubrano V, Vassalle C, L'Abbate A, Zucchelli GC. A new method to evaluate oxidative stress in humans. Immunoanal Biol Spec. 2002; 17:172–175.17. Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011; 34:1041–1045.18. Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 172:994–1001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Cardiopulmonary Function in the Patients with Ankylosing Spondylitis Using Exercise Stress Test
- Effect and Safety of Cardiac Rehabilitation Program in Heart Failure
- Cardiac Rehabilitation After Acute Myocardial Infarction Resuscitated From Cardiac Arrest
- The Effect of a Self Exercise Program in Cardiac Rehabilitation for Patients with Coronary Artery Disease
- The Effect of Individualized Exercise Parameters Applied to Two Patients Recovering from Implanted Left Ventricular Assist Devices in Korea