J Korean Surg Soc.
2012 Nov;83(5):298-306. 10.4174/jkss.2012.83.5.298.
Immediately transcripted genes in various hepatic ischemia models
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Surgery, Gachon University of Medicine and Science, Incheon, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Graduate Program of Nano Science and Technology, Graduate School of Yonsei University, Seoul, Korea.
- 5Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. kskim88@yuhs.ac
- KMID: 2145007
- DOI: http://doi.org/10.4174/jkss.2012.83.5.298
Abstract
- PURPOSE
To elucidate the characteristic gene transcription profiles among various hepatic ischemia conditions, immediately transcribed genes and the degree of ischemic injury were compared among total ischemia (TI), intermittent clamping (IC), and ischemic preconditioning (IPC).
METHODS
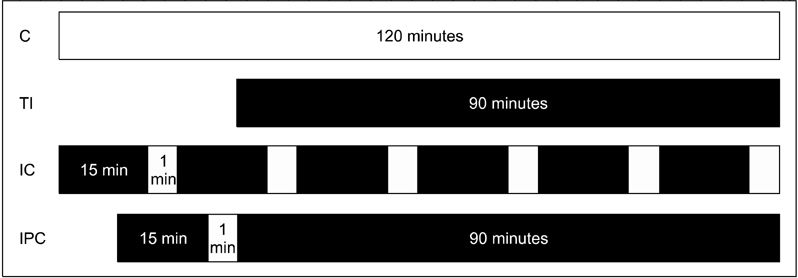
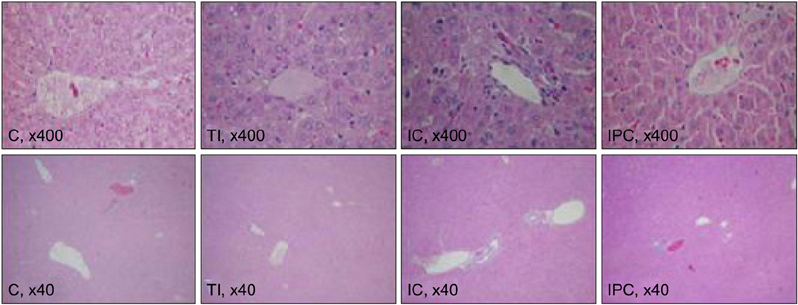
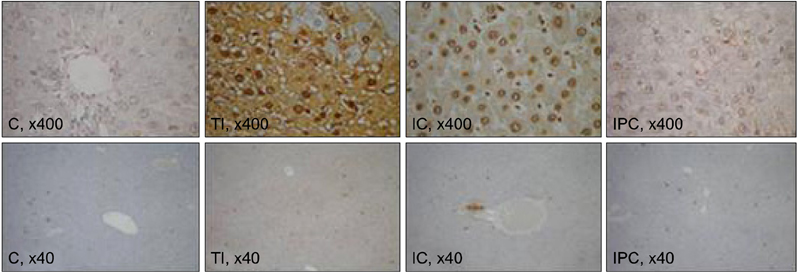
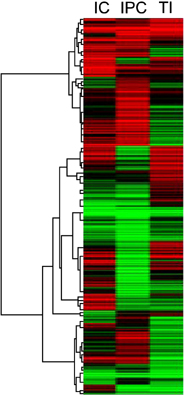
Sprague-Dawley rats were equally divided into control (C, sham-operated), TI (ischemia for 90 minutes), IC (ischemia for 15 minutes and reperfusion for 5 minutes, repeated six times), and IPC (ischemia for 15 minutes, reperfusion for 5 minutes, and ischemia again for 90 minutes) groups. A cDNA microarray analysis was performed using hepatic tissues obtained by partial hepatectomy after occluding hepatic inflow.
RESULTS
The cDNA microarray revealed the following: interleukin (IL)-1beta expression was 2-fold greater in the TI group than in the C group. In the IC group, IL-1alpha/beta expression increased by 2.5-fold, and Na+/K+ ATPase beta1 expression decreased by 2.4-fold. In the IPC group, interferon regulatory factor-1, osteoprotegerin, and retinoblastoma-1 expression increased by approximately 2-fold compared to that in the C group, but the expression of Na+/K+ ATPase beta1 decreased 3-fold.
CONCLUSION
The current findings revealed characteristic gene expression profiles under various ischemic conditions. However, additional studies are needed to clarify the mechanism of protection against IPC.
MeSH Terms
-
Adenosine Triphosphatases
Apoptosis
Constriction
Hepatectomy
Interferon Regulatory Factor-1
Interleukins
Ischemia
Ischemic Preconditioning
Microarray Analysis
Necrosis
Oligonucleotide Array Sequence Analysis
Osteoprotegerin
Rats, Sprague-Dawley
Reperfusion
Reperfusion Injury
Transcriptome
Adenosine Triphosphatases
Interferon Regulatory Factor-1
Interleukins
Osteoprotegerin
Figure
Reference
-
1. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986. 74:1124–1136.2. Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006. 244:921–928.3. Clavien PA, Selzner M, Rudiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003. 238:843–850.4. Fernandez L, Carrasco-Chaumel E, Serafín A, Xaus C, Grande L, Rimola A, et al. Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am J Transplant. 2004. 4:888–899.5. Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004. 40:933–941.6. Koti RS, Yang W, Glantzounis G, Quaglia A, Davidson BR, Seifalian AM. Effect of ischaemic preconditioning on hepatic oxygenation, microcirculation and function in a rat model of moderate hepatic steatosis. Clin Sci (Lond). 2005. 108:55–63.7. Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003. 39:55–61.8. Serafín A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002. 161:587–601.9. Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall'Agata M, et al. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 2001. 33:808–815.10. Barrier A, Olaya N, Chiappini F, Roser F, Scatton O, Artus C, et al. Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J. 2005. 19:1617–1626.11. Navarro-Sabate A, Peralta C, Calvo MN, Manzano A, Massip-Salcedo M, Rosello-Catafau J, et al. Mediators of rat ischemic hepatic preconditioning after cold preservation identified by microarray analysis. Liver Transpl. 2006. 12:1615–1625.12. Butte A. The use and analysis of microarray data. Nat Rev Drug Discov. 2002. 1:951–960.13. Bilbao G, Contreras JL, Eckhoff DE, Mikheeva G, Krasnykh V, Douglas JT, et al. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999. 230:185–193.14. Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998. 27:1652–1660.15. Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001. 33:397–405.16. Jin LM, Liu YX, Zhou L, Xie HY, Feng XW, Li H, et al. Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats. Pathobiology. 2010. 77:136–146.17. Pan W, Lin J, Le CT. How many replicates of arrays are required to detect gene expression changes in microarray experiments? A mixture model approach. Genome Biol. 2002. 3:research0022.18. Ishii S, Abe T, Saito T, Tsuchiya T, Kanno H, Miyazawa M, et al. Effects of preconditioning on ischemia/reperfusion injury of hepatocytes determined by immediate early gene transcription. J Hepatobiliary Pancreat Surg. 2001. 8:461–468.19. Jang JH, Kang KJ, Kang Y, Lee IS, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transpl. 2008. 14:980–988.20. Banga NR, Homer-Vanniasinkam S, Graham A, Al-Mukhtar A, White SA, Prasad KR. Ischaemic preconditioning in transplantation and major resection of the liver. Br J Surg. 2005. 92:528–538.21. Chen W, Qiu JF, Zhang ZQ, Luo HF, Rosello-Catafau J, Wu ZY. Gene expression changes after hypoxic preconditioning in rat hepatocytes. Hepatobiliary Pancreat Dis Int. 2006. 5:416–421.22. Raza A, Dikdan G, Desai KK, Shareef A, Fernandes H, Aris V, et al. Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transpl. 2010. 16:588–599.23. Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, et al. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000. 279:G163–G171.24. Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004. 39:1533–1543.25. Jaeschke H. Reperfusion injury after warm ischemia or cold storage of the liver: role of apoptotic cell death. Transplant Proc. 2002. 34:2656–2658.26. Goodrich DW. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene. 2006. 25:5233–5243.27. Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997. 99:2930–2940.28. Fili S, Karalaki M, Schaller B. Therapeutic implications of osteoprotegerin. Cancer Cell Int. 2009. 9:26.29. Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010. 55:2049–2061.30. Jensen JK, Ueland T, Atar D, Gullestad L, Mickley H, Aukrust P, et al. Osteoprotegerin concentrations and prognosis in acute ischaemic stroke. J Intern Med. 2010. 267:410–417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Experimental Study in Mice for Inducing the Optimal Hepatic Ischemia Followed by Reperfusion
- Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review
- Hepatic Infarction following Hepatic Artery Embolization for Iatrogenic Hepatic Arterial Hemorrhage
- Animal Models of Acute Hepatic Failure
- Animal Model of Cerebral Ischemia