J Korean Soc Magn Reson Med.
2013 Dec;17(4):259-266. 10.13104/jksmrm.2013.17.4.259.
Pharmacokinetics and Bio-distribution of New Gd-complexes of DTPA-bis (amide) (L3) in a Rat Model
- Affiliations
-
- 1Department of Radiology, the Second Affiliated Hospital, Shantou University Medical College, Shantou 515041, PR China.
- 2Department of Radiology & Molecular Medicine, Kyungpook National University, Daegu, Korea. ychang@knu.ac.kr
- 3Department of Radiology, Bogang Hospital, Daegu, Korea.
- KMID: 2144327
- DOI: http://doi.org/10.13104/jksmrm.2013.17.4.259
Abstract
- PURPOSE
To investigate the blood pharmacokinetics and bio-distribution of DTPA-bis-amide (L3) Gd(III) complexes.
MATERIALS AND METHODS
The pharmacokinetics and bio-distribution of Gd (L3)(H2O).nH2O were investigated in Sprague-Dawley rats after intravenous administration at a dose of 0.1 mmol Gd/kg. The Gd content in the blood, various tissues, and organs was determined by ICP-AES. Blood pharmacokinetic parameters were calculated using a two-compartment model.
RESULTS
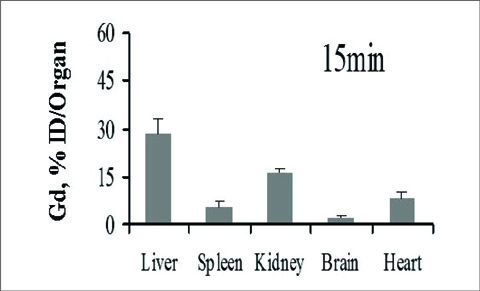
The half-lives of alphaphase and betaphase Gd (L3)(H2O).nH2O were 2.286+/-0.11 min and 146.1+/-7.5 min, respectively. The bio-distribution properties reveal that the complex is mainly excreted by the renal pathway, and possibly excreted by the hepatobiliary route. The concentration ratio of Gd (III) was significantly higher in the liver and spleen than in other organs, and small amounts of Gd (III) ion were detected in the blood or other tissues of rats only after 7 days of intravenous administration.
CONCLUSION
The MRI contrast agent Gd (L3)(H2O).nH2O provides prolonged blood pool retention in the circulation and then clears rapidly with minimal accumulation of Gd(III) ions. The synthesis of gadolinium complexes with well-balanced lipophilicity and hydrophilicity shows promise for their further development as blood pool MRI contrast agents.
Keyword
MeSH Terms
Figure
Reference
-
1. Mansfield P. Nmr imaging in biomedicine: Supplement 2 advances in magnetic resonance. 1982. Access Online via Elsevier.2. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MRI single-subject brain. Neuroimage. 2002; 15:273–289.3. Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996; 36:715–725.4. Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.5. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium (iii) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999; 99:2293–2352.6. Jacques V, Desreux JF. New classes of MRI contrast agents. Contrast agents i. Springer;2002. p. 123–164.7. Aime S, Botta M, Terreno E. Gd (iii)-based contrast agents for MRI. Adv Inorg Chem. 2005; 57:173–237.8. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997; 7:91–101.9. Hamm B, Staks T, Muühler A, et al. Phase i clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: Safety, pharmacokinetics, and MR imaging. Radiology. 1995; 195:785–792.10. Kobayashi H, Kawamoto S, Jo SK, Bryant HL Jr, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem. 2003; 14:388–394.11. Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006; 35:512–523.12. Dutta S, Park JA, Jung JC, Chang Y, Kim TJ. Gd-complexes of DTPA-bis (amide) conjugates of tranexamic acid and its esters with high relaxivity and stability for magnetic resonance imaging. Dalton Trans. 2008; 28:2199–2206.13. Gu S, Kim HK, Lee GH, Kang BS, Chang Y, Kim TJ. Gd-complexes of 1, 4, 7, 10-tetraazacyclododecane-n, n', n'', n'''-1, 4, 7, 10-tetraacetic acid (DOTA) conjugates of tranexamates as a new class of blood-pool magnetic resonance imaging contrast agents. J Med Chem. 2011; 54:143–152.14. Wedeking P, Kumar K, Tweedle M. Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging. 1992; 10:641–648.15. Parmelee DJ, Walovitch RC, Ouellet HS, Lauffer RB. Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest Radiol. 1997; 32:741–747.16. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses gd-eob-dtpa (gadoxetic acid) with standard gddtpa. Invest Radiol. 2009; 44:305–310.17. Fasano M, Curry S, Terreno E, et al. The extraordinary ligand binding properties of human serum albumin. IUBMB life. 2005; 57:787–796.18. Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using mri. Magn Reson Med. 2012; 67:769–777.19. Borlongan CV, Emerich DF. Facilitation of drug entry into the cns via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, cereport. Brain Res Bull. 2003; 60:297–306.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Magnetic Relaxation Properties of DTPA-bis(4-carboxycyclohexyl) amide Paramagnetic Gd-chelates
- Clinical experience of adverse drug reaction in gadolinium-DTPA enhancement of MRI
- Visualization of Tumor Angiogenesis Using MR Imaging Contrast Agent Gd-DTPA-anti-VEGF Receptor 2 Antibody Conjugate in a Mouse Tumor Model
- Effect of Gd-DTPA on Diffusion in Canine Brain with Hyperacute Stroke
- Enhancement Pattern of Liver Parenchyma during Late Dynamic Phase Imaging: Comparison between Gd-EOB-DTPA and Gd-DTPA-BMA