Korean J Radiol.
2010 Aug;11(4):449-456. 10.3348/kjr.2010.11.4.449.
Visualization of Tumor Angiogenesis Using MR Imaging Contrast Agent Gd-DTPA-anti-VEGF Receptor 2 Antibody Conjugate in a Mouse Tumor Model
- Affiliations
-
- 1Institute for Radiological Imaging Science, Wonkwang University School of Medicine, Jeonbuk 570-711, Korea. khy1646@wonkwang.ac.kr
- 2Department of Radiology, Wonkwang University School of Medicine, Jeonbuk 570-711, Korea.
- 3Department of Pathology, Wonkwang University School of Medicine, Jeonbuk 570-711, Korea.
- KMID: 984899
- DOI: http://doi.org/10.3348/kjr.2010.11.4.449
Abstract
OBJECTIVE
To visualize tumor angiogenesis using the MRI contrast agent, Gd-DTPA-anti-VEGF receptor 2 antibody conjugate, with a 4.7-Tesla MRI instrument in a mouse model.
MATERIALS AND METHODS
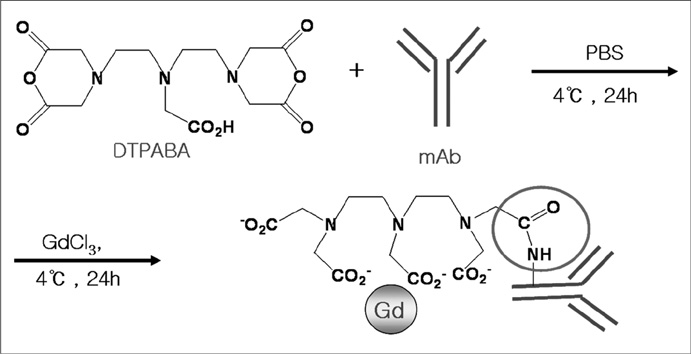
We designed a tumor angiogenesis-targeting T1 contrast agent that was prepared by the bioconjugation of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) and an anti-vascular endothelial growth factor receptor-2 (VEGFR2) antibody. The specific binding of the agent complex to cells that express VEGFR2 was examined in cultured murine endothelial cells (MS-1 cells) with a 4.7-Tesla magnetic resonance imaging scanner. Angiogenesis-specific T1 enhancement was imaged with the Gd-DTPA-anti-VEGFR2 antibody conjugate using a CT-26 adenocarcinoma tumor model in eight mice. As a control, the use of the Gd-DTPA-anti-rat immunoglobulin G (Gd-DTPA-anti-rat IgG) was imaged with a tumor model in eight mice. Statistical significance was assessed using the Mann-Whitney test. Tumor tissue was examined by immunohistochemical analysis.
RESULTS
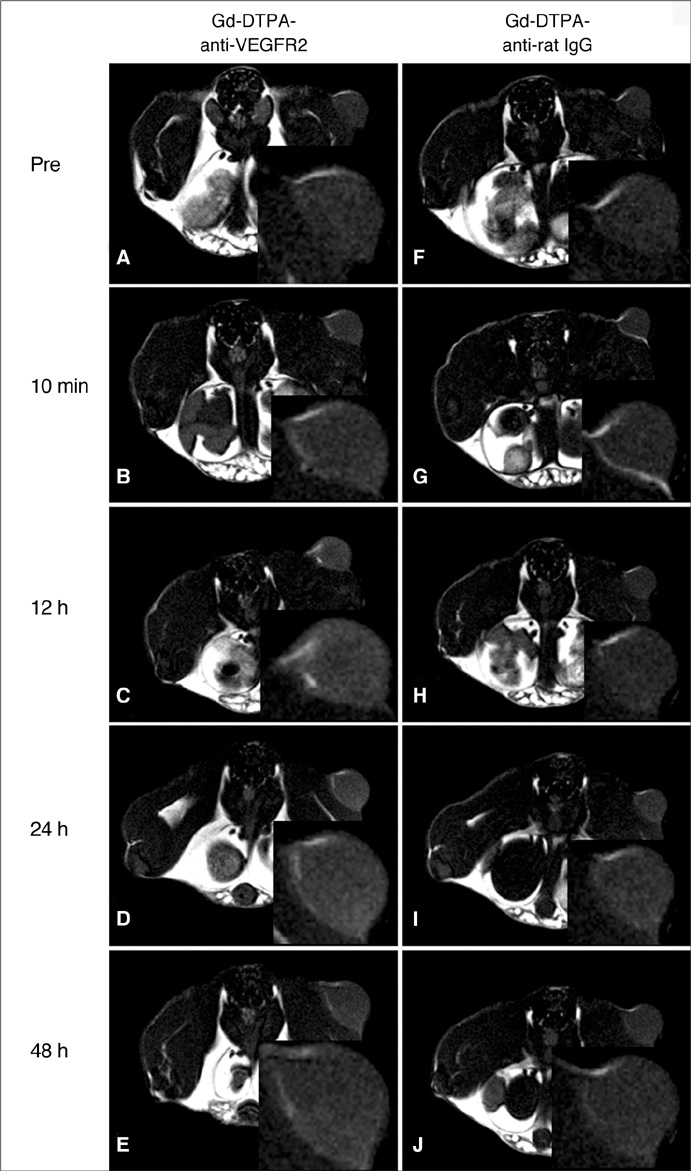
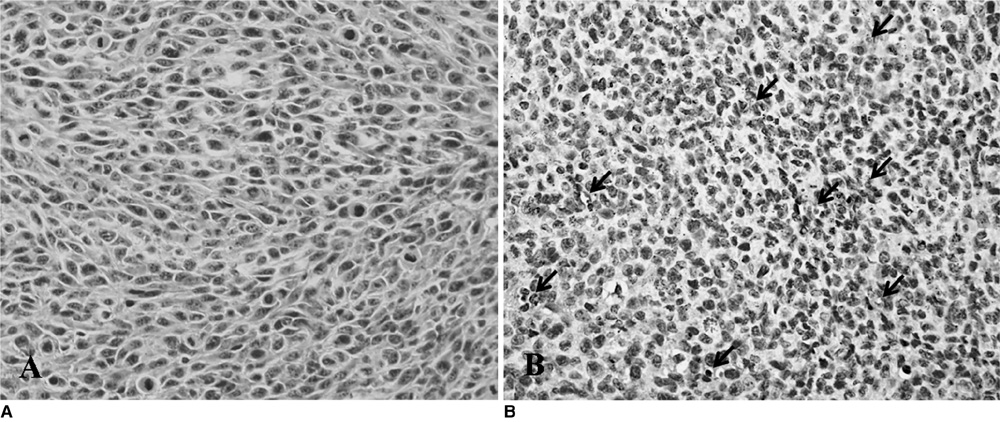
The Gd-DTPA-anti-VEGFR2 antibody conjugate showed predominant binding to cultured endothelial cells that expressed a high level of VEGFR2. Signal enhancement was approximately three-fold for in vivo T1-weighted MR imaging with the use of the Gd-DTPA-anti-VEGFR2 antibody conjugate as compared with the Gd-DTPA-rat IgG in the mouse tumor model (p < 0.05). VEGFR2 expression in CT-26 tumor vessels was demonstrated using immunohistochemical staining.
CONCLUSION
MR imaging using the Gd-DTPA-anti-VEGFR2 antibody conjugate as a contrast agent is useful in visualizing noninvasively tumor angiogenesis in a murine tumor model.
MeSH Terms
-
Adenocarcinoma/*pathology
Animals
Colonic Neoplasms/*pathology
Contrast Media/chemistry/*diagnostic use
Gadolinium DTPA/chemistry/*diagnostic use
Immunoenzyme Techniques
Magnetic Resonance Imaging/*methods
Mice
Mice, Nude
Neovascularization, Pathologic/*diagnosis
Rats
Statistics, Nonparametric
Tumor Cells, Cultured
Vascular Endothelial Growth Factor Receptor-2/*antagonists & inhibitors/chemistry
Figure
Reference
-
1. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.2. Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000. 89:488–499.3. Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999. 77:527–543.4. Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995. 26:86–91.5. Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995. 95:1789–1797.6. Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995. 87:506–516.7. Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993. 91:153–159.8. Brasch R, Turetschek K. MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol. 2000. 34:148–155.9. Zhang D, Feng XY, Henning TD, Wen L, Lu WY, Pan H, et al. MR imaging of tumor angiogenesis using sterically stabilized Gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2009. 70:180–189.10. Li KC, Bednarski MD. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J Magn Reson Imaging. 2002. 16:388–393.11. Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998. 4:623–626.12. Sipkins DA, Gijbels K, Tropper FD, Bednarski M, Li KC, Steinman L. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol. 2000. 104:1–9.13. Poduslo JF, Curran GL, Peterson JA, McCormick DJ, Fauq AH, Khan MA, et al. Design and chemical synthesis of a magnetic resonance contrast agent with enhanced in vitro binding, high blood-brain barrier permeability, and in vivo targeting to Alzheimer's disease amyloid plaques. Biochemistry. 2004. 43:6064–6075.14. Choi KS, Kim SH, Cai QY, Kim SY, Kim HO, Lee HJ, et al. Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol Imaging. 2007. 6:75–84.15. Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007. 38:258–268.16. Luo JC, Yamaguchi S, Shinkai A, Shitara K, Shibuya M. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 1998. 58:2652–2660.17. Nagy JA, Masse EM, Herzberg KT, Meyers MS, Yeo KT, Yeo TK, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995. 55:360–368.18. Rosen LS. VEGF-targeted therapy: therapeutic potential and recent advances. Oncologist. 2005. 10:382–391.19. Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998. 58:2594–2600.20. Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006. 39:469–478.21. Ran S, Huang X, Downes A, Thorpe PE. Evaluation of novel antimouse VEGFR2 antibodies as potential antiangiogenic or vascular targeting agents for tumor therapy. Neoplasia. 2003. 5:297–307.22. Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007. 9:415–447.23. Bekeredjian R, Katus HA, Kuecherer HF. Therapeutic use of ultrasound targeted microbubble destruction: a review of non-cardiac applications. Ultraschall Med. 2006. 27:134–140.24. Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001. 104:2107–2112.25. Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007. 116:276–284.26. Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, et al. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res. 2002. 62:6146–6151.27. Lu E, Wagner WR, Schellenberger U, Abraham JA, Klibanov AL, Woulfe SR, et al. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003. 108:97–103.28. Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007. 188:11–23.29. Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol. 2005. 205:194–205.30. Lee SI, Lee SY, Yoon KH, Choi KS, Jang KY, Yoo WH, et al. Molecular MR imaging for visualizing ICAM-1 expression in the inflamed synovium of collagen-induced arthritic mice. Korean J Radiol. 2009. 10:472–480.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supradiaphragmatic Liver Confirmed by a Hepatocyte-specific Contrast Agent (Gd-EOB-DTPA): A Case Report
- Comparison of Gadolinium Polylysine and Gadopentetate in Contrast Enhanced MR Imaging of IVlyocardial Ischemia-Reperfusion in Cats
- Diagnostic Significance of pH-Responsive Gd³âº-Based Tâ‚ MR Contrast Agents
- Clinical experience of adverse drug reaction in gadolinium-DTPA enhancement of MRI
- Enhancement Pattern of Liver Parenchyma during Late Dynamic Phase Imaging: Comparison between Gd-EOB-DTPA and Gd-DTPA-BMA