J Clin Neurol.
2010 Mar;6(1):1-9. 10.3988/jcn.2010.6.1.1.
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy: A Genetic Cause of Cerebral Small Vessel Disease
- Affiliations
-
- 1Department of Neurology and Institute of Medical Science, Jeju National University School of Medicine, Jeju, Korea. iguazzu@hanmail.net
- KMID: 2135475
- DOI: http://doi.org/10.3988/jcn.2010.6.1.1
Abstract
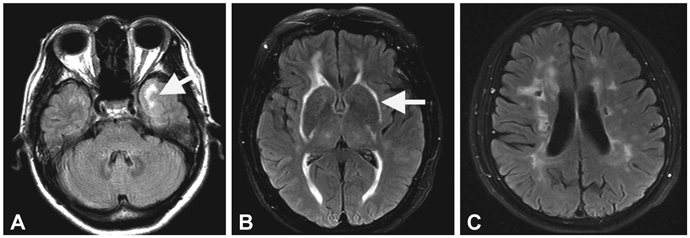
- Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a single-gene disorder of the cerebral small blood vessels caused by mutations in the Notch3 gene. The exact prevalence of this disorder was unknown currently, and the number of reported CADASIL families is steadily increasing as the clinical picture and diagnostic examinations are becoming more widely known. The main clinical manifestations are recurrent stroke, migraine, psychiatric symptoms, and progressive cognitive impairment. The clinical course of CADASIL is highly variable, even within families. The involvement of the anterior temporal lobe and the external capsule on brain magnetic resonance imaging was found to have high sensitivity and specificity in differentiating CADASIL from the much more common sporadic cerebral small-vessel disease (SVD). The pathologic hallmark of the disease is the presence of granular osmiophilic material in the walls of affected vessels. CADASIL is a prototype single-gene disorder that has evolved as a unique model for studying the mechanisms underlying cerebral SVD. At present, the incidence and prevalence of CADASIL seem to be underestimated due to limitations in clinical, neuroradiological, and genetic diagnoses of this disorder.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
A Case of Transient Memory Impairment after Acute Left Focal Lateral Putamen ICH with Old Caudate Nucleus Infarction
Chang Woon Choi, Chan-Nyoung Lee, Kun Woo Park
Dement Neurocogn Disord. 2012;11(4):154-157. doi: 10.12779/dnd.2012.11.4.154.A Case of Transient Memory Impairment after Acute Left Focal Lateral Putamen ICH with Old Caudate Nucleus Infarction
Chang Woon Choi, Chan-Nyoung Lee, Kun Woo Park
Dement Neurocogn Disord. 2012;11(4):154-157. doi: 10.12779/dnd.2012.11.4.154.
Reference
-
1. Ringelstein EB, Nabavi DG. Cerebral small vessel diseases: cerebral microangiopathies. Curr Opin Neurol. 2005. 18:179–188.
Article2. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996. 383:707–710.
Article3. Bousser MG, Tournier-Lasserve E. Summary of the proceedings of the First International Workshop on CADASIL. Paris, May 19-21, 1993. Stroke. 1994. 25:704–707.
Article4. Sourander P, Walinder J. Hereditary multi-infarct dementia. Morphological and clinical studies of a new disease. Acta Neuropathol. 1977. 39:247–254.5. Stevens DL, Hewlett RH, Brownell B. Chronic familial vascular encephalopathy. Lancet. 1977. 1:1364–1365.
Article6. Low WC, Junna M, Borjesson-Hanson A, Morris CM, Moss TH, Stevens DL, et al. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain. 2007. 130(Pt 2):357–367.
Article7. Tournier-Lasserve E, Joutel A, Melki J, Weissenbach J, Lathrop GM, Chabriat H, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12. Nat Genet. 1993. 3:256–259.
Article8. Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997. 350:1511–1515.
Article9. Razvi SS, Davidson R, Bone I, Muir KW. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. J Neurol Neurosurg Psychiatry. 2005. 76:739–741.
Article10. Dong Y, Hassan A, Zhang Z, Huber D, Dalageorgou C, Markus HS. Yield of screening for CADASIL mutations in lacunar stroke and leukoaraiosis. Stroke. 2003. 34:203–205.
Article11. Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet. 1995. 346:934–939.12. Dichgans M, Mayer M, Uttner I, Bruning R, Muller-Hocker J, Rungger G, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol. 1998. 44:731–739.
Article13. van den Boom R, Lesnik Oberstein SA, Ferrari MD, Haan J, van Buchem MA. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR imaging findings at different ages-3rd-6th decades. Radiology. 2003. 229:683–690.
Article14. Mykkanen K, Savontaus ML, Juvonen V, Sistonen P, Tuisku S, Tuominen S, et al. Detection of the founder effect in Finnish CADASIL families. Eur J Hum Genet. 2004. 12:813–819.
Article15. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004. 127(Pt 11):2533–2539.
Article16. Singhal S, Bevan S, Barrick T, Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004. 127(Pt 9):2031–2038.
Article17. Dotti MT, Federico A, Mazzei R, Bianchi S, Scali O, Conforti FL, et al. The spectrum of Notch3 mutations in 28 Italian CADASIL families. J Neurol Neurosurg Psychiatry. 2005. 76:736–738.
Article18. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999. 284:770–776.
Article19. Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000. 105:597–605.
Article20. Alva JA, Iruela-Arispe ML. Notch signaling in vascular morphogenesis. Curr Opin Hematol. 2004. 11:278–283.
Article21. Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling pathway. Am J Hum Genet. 2004. 74:338–347.
Article22. Monet-Lepretre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, et al. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain. 2009. 132(Pt 6):1601–1612.
Article23. Opherk C, Duering M, Peters N, Karpinska A, Rosner S, Schneider E, et al. CADASIL mutations enhance spontaneous multimerization of NOTCH3. Hum Mol Genet. 2009. 18:2761–2767.
Article24. Tikka S, Mykkanen K, Ruchoux MM, Bergholm R, Junna M, Poyhonen M, et al. Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain. 2009. 132(Pt 4):933–939.
Article25. Kim Y, Kim JS, Kim G, No YJ, Yoo HW. Two novel mutations of the NOTCH3 gene in Korean patients with CADASIL. Mutat Res. 2006. 593:116–120.
Article26. Kim Y, Choi EJ, Choi CG, Kim G, Choi JH, Yoo HW, et al. Characteristics of CADASIL in Korea: a novel cysteine-sparing Notch3 mutation. Neurology. 2006. 66:1511–1516.
Article27. Mazzei R, Conforti FL, Lanza PL, Sprovieri T, Lupo MR, Gallo O, et al. A novel Notch3 gene mutation not involving a cysteine residue in an Italian family with CADASIL. Neurology. 2004. 63:561–564.
Article28. Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case. Stroke. 2002. 33:2565–2569.
Article29. Scheid R, Heinritz W, Leyhe T, Thal DR, Schober R, Strenge S, et al. Cysteine-sparing notch3 mutations: cadasil or cadasil variants? Neurology. 2008. 71:774–776.
Article30. Coto E, Menendez M, Navarro R, Garcia-Castro M, Alvarez V. A new de novo Notch3 mutation causing CADASIL. Eur J Neurol. 2006. 13:628–631.
Article31. Joutel A, Dodick DD, Parisi JE, Cecillon M, Tournier-Lasserve E, Bousser MG. De novo mutation in the Notch3 gene causing CADASIL. Ann Neurol. 2000. 47:388–391.32. Tuominen S, Juvonen V, Amberla K, Jolma T, Rinne JO, Tuisku S, et al. Phenotype of a homozygous CADASIL patient in comparison to 9 age-matched heterozygous patients with the same R133C Notch3 mutation. Stroke. 2001. 32:1767–1774.
Article33. Lee YC, Liu CS, Chang MH, Lin KP, Fuh JL, Lu YC, et al. Population-specific spectrum of NOTCH3 mutations, MRI features and founder effect of CADASIL in Chinese. J Neurol. 2009. 256:249–255.
Article34. Kang SY, Oh JH, Kang JH, Choi JC, Lee JS. Nerve conduction studies in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neurol. 2009. 256:1724–1727.
Article35. Fouillade C, Chabriat H, Riant F, Mine M, Arnoud M, Magy L, et al. Activating NOTCH3 mutation in a patient with small-vessel-disease of the brain. Hum Mutat. 2008. 29:452.
Article36. Ruchoux MM, Maurage CA. CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997. 56:947–964.37. Viswanathan A, Gray F, Bousser MG, Baudrimont M, Chabriat H. Cortical neuronal apoptosis in CADASIL. Stroke. 2006. 37:2690–2695.
Article38. Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004. 14:358–364.
Article39. Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, et al. Arterioles of the lenticular nucleus in CADASIL. Stroke. 2006. 37:2242–2247.
Article40. Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser MG. Autosomal dominant leukoencephalopathy and subcortical ischemic stroke. A clinicopathological study. Stroke. 1993. 24:122–125.
Article41. Ishiko A, Shimizu A, Nagata E, Takahashi K, Tabira T, Suzuki N. Notch3 ectodomain is a major component of granular osmiophilic material (GOM) in CADASIL. Acta Neuropathol. 2006. 112:333–339.
Article42. Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol. 1995. 89:500–512.
Article43. Ruchoux MM, Chabriat H, Bousser MG, Baudrimont M, Tournier-Lasserve E. Presence of ultrastructural arterial lesions in muscle and skin vessels of patients with CADASIL. Stroke. 1994. 25:2291–2292.
Article44. Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr JP. The natural history of CADASIL: a pooled analysis of previously published cases. Stroke. 1999. 30:1230–1233.45. Choi EJ, Choi CG, Kim JS. Large cerebral artery involvement in CADASIL. Neurology. 2005. 65:1322–1324.
Article46. Choi JC, Kang SY, Kang JH, Park JK. Intracerebral hemorrhages in CADASIL. Neurology. 2006. 67:2042–2044.
Article47. Peters N, Herzog J, Opherk C, Dichgans M. A two-year clinical follow-up study in 80 CADASIL subjects: progression patterns and implications for clinical trials. Stroke. 2004. 35:1603–1608.
Article48. Buffon F, Porcher R, Hernandez K, Kurtz A, Pointeau S, Vahedi K, et al. Cognitive profile in CADASIL. J Neurol Neurosurg Psychiatry. 2006. 77:175–180.
Article49. Charlton RA, Morris RG, Nitkunan A, Markus HS. The cognitive profiles of CADASIL and sporadic small vessel disease. Neurology. 2006. 66:1523–1526.
Article50. Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, et al. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007. 38:923–928.
Article51. Santa Y, Uyama E, Chui DH, Arima M, Kotorii S, Takahashi K, et al. Genetic, clinical and pathological studies of CADASIL in Japan: a partial contribution of Notch3 mutations and implications of smooth muscle cell degeneration for the pathogenesis. J Neurol Sci. 2003. 212:79–84.
Article52. Valenti R, Poggesi A, Pescini F, Inzitari D, Pantoni L. Psychiatric disturbances in CADASIL: a brief review. Acta Neurol Scand. 2008. 118:291–295.
Article53. Reyes S, Viswanathan A, Godin O, Dufouil C, Benisty S, Hernandez K, et al. Apathy: a major symptom in CADASIL. Neurology. 2009. 72:905–910.
Article54. Zicari E, Tassi R, Stromillo ML, Pellegrini M, Bianchi S, Cevenini G, et al. Right-to-left shunt in CADASIL patients: prevalence and correlation with clinical and MRI findings. Stroke. 2008. 39:2155–2157.55. Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, et al. Patterns of MRI lesions in CADASIL. Neurology. 1998. 51:452–457.
Article56. O'Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001. 56:628–634.57. Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O'Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007. 69:172–179.
Article58. Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006. 129(Pt 9):2375–2383.
Article59. Holtmannspotter M, Peters N, Opherk C, Martin D, Herzog J, Bruckmann H, et al. Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study. Stroke. 2005. 36:2559–2565.
Article60. Peters N, Holtmannspotter M, Opherk C, Gschwendtner A, Herzog J, Samann P, et al. Brain volume changes in CADASIL: a serial MRI study in pure subcortical ischemic vascular disease. Neurology. 2006. 66:1517–1522.
Article61. Mellies JK, Baumer T, Muller JA, Tournier-Lasserve E, Chabriat H, Knobloch O, et al. SPECT study of a German CADASIL family: a phenotype with migraine and progressive dementia only. Neurology. 1998. 50:1715–1721.
Article62. Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, et al. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000. 31:1904–1912.
Article63. Tuominen S, Miao Q, Kurki T, Tuisku S, Poyhonen M, Kalimo H, et al. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. 2004. 35:1063–1067.
Article64. Razvi SS, Davidson R, Bone I, Muir KW. Is inadequate family history a barrier to diagnosis in CADASIL? Acta Neurol Scand. 2005. 112:323–326.
Article65. Ebke M, Dichgans M, Bergmann M, Voelter HU, Rieger P, Gasser T, et al. CADASIL: skin biopsy allows diagnosis in early stages. Acta Neurol Scand. 1997. 95:351–357.
Article66. Markus HS, Martin RJ, Simpson MA, Dong YB, Ali N, Crosby AH, et al. Diagnostic strategies in CADASIL. Neurology. 2002. 59:1134–1138.
Article67. Joutel A, Favrole P, Labauge P, Chabriat H, Lescoat C, Andreux F, et al. Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet. 2001. 358:2049–2051.
Article68. Forteza AM, Brozman B, Rabinstein AA, Romano JG, Bradley WG. Acetazolamide for the treatment of migraine with aura in CADASIL. Neurology. 2001. 57:2144–2145.
Article69. Weller M, Dichgans J, Klockgether T. Acetazolamide-responsive migraine in CADASIL. Neurology. 1998. 50:1505.
Article70. Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008. 7:310–318.
Article71. Dichgans M, Petersen D. Angiographic complications in CADASIL. Lancet. 1997. 349:776–777.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Patients with R544C Mutation Aged 90 or Older
- Genetics of Cerebral Small Vessel Disease
- A Case of CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) Diagnosed by Skin Biopsy
- Neuroimaging Characteristics of Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) in Korean Based on Jeju Cohort: A Pictorial Essay
- Phenotypic Features of Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Subjects with R544C Mutation