Korean J Ophthalmol.
2014 Jun;28(3):257-264. 10.3341/kjo.2014.28.3.257.
Topical Prostaglandin Analogue Drugs Inhibit Adipocyte Differentiation
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2133325
- DOI: http://doi.org/10.3341/kjo.2014.28.3.257
Abstract
- PURPOSE
To investigate the effects of topical prostaglandin analogue drugs on the differentiation of adipocytes.
METHODS
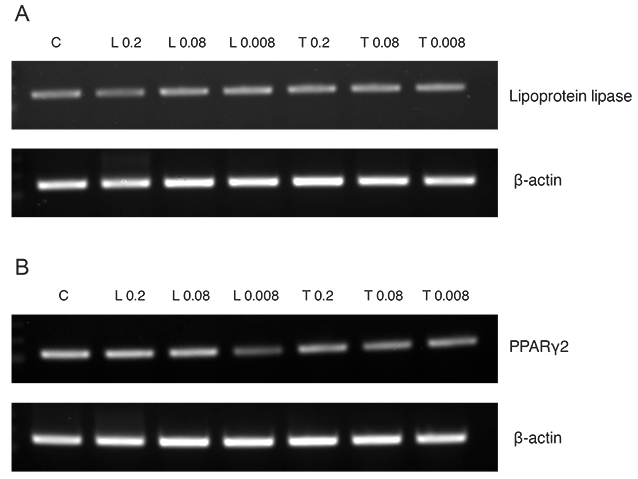
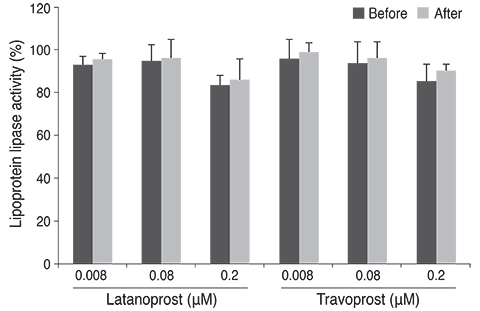
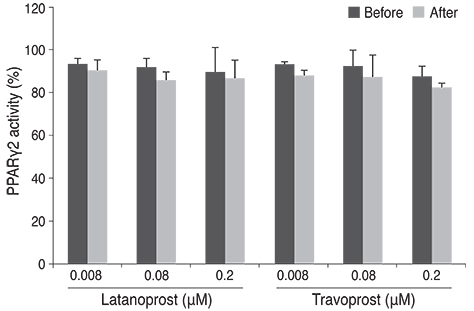
Differentiation of 3T3-L1 preadipocytes was induced with isobutylmethylxanthine, dexamethasone, and insulin. 3T3-L1 cells were exposed to 0.008, 0.08, 0.2 microM of latanoprost and travoprost. Reverse transcription polymerase chain reaction for mRNA expression of lipoprotein lipase and peroxisome proliferator-activated receptor gamma 2 (PPARgamma2), and glycerol-3-phosphate dehydrogenase (G3PDH) assays were performed to examine the effects on early and late differentiation, respectively. Also, glycerol assays were done to evaluate the effect of prostaglandin analogues on lipolysis after differentiation.
RESULTS
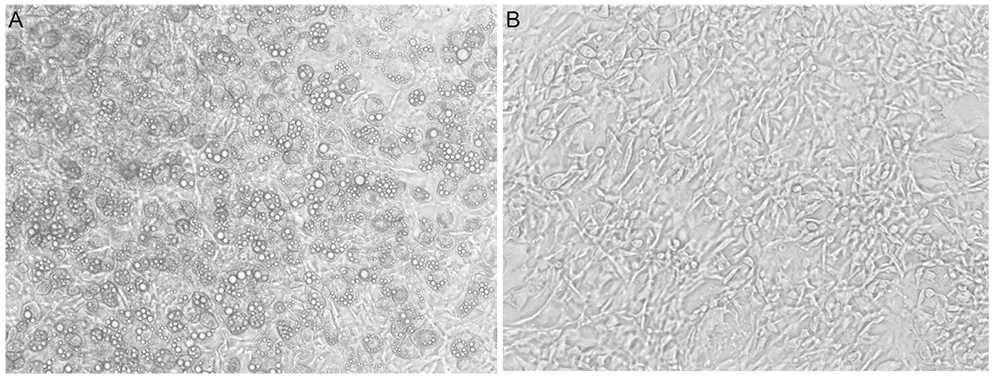
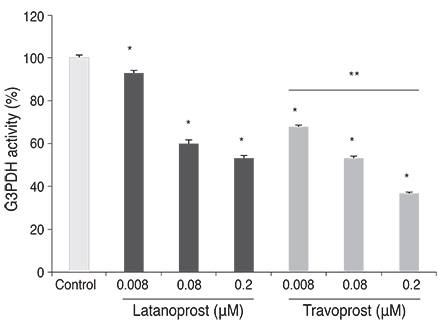
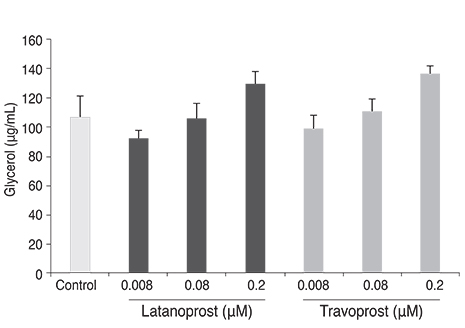
Both prostaglandin analogues inhibited differentiation of preadipocytes. Topical prostaglandin analogues significantly decreased G3PDH activity, a marker of late differentiation. However, topical prostaglandin analogues did not change mRNA expressions of lipoprotein lipase and PPARgamma2, markers of early differentiation. The activities of the early markers of differentiation were not changed significantly before and after growth arrest. Compared to latanoprost, travoprost decreased G3PDH activity more significantly (p < 0.05). Both prostaglandin analogues did not affect the lipolysis of differentiated adipocytes (p > 0.05).
CONCLUSIONS
Prostaglandin analogues display an inhibitory effect on the differentiation of adipocytes when the cells start to differentiate especially in the late stage of differentiation. Thus, commercial topical prostaglandin analogues may decrease the fat contents of eyelids.
Keyword
MeSH Terms
-
3T3-L1 Cells
Adipocytes/drug effects/*pathology
Animals
Antihypertensive Agents/administration & dosage
Cell Differentiation/drug effects
Disease Models, Animal
Glaucoma/*drug therapy/pathology
Lipolysis/*drug effects
Mice
Neuroprotective Agents/administration & dosage
Ophthalmic Solutions/administration & dosage
Prostaglandins F, Synthetic/*administration & dosage
Prostaglandins, Synthetic/*administration & dosage
Antihypertensive Agents
Neuroprotective Agents
Ophthalmic Solutions
Prostaglandins F, Synthetic
Prostaglandins, Synthetic
Figure
Reference
-
1. Krauss AH, Woodward DF. Update on the mechanism of action of bimatoprost: a review and discussion of new evidence. Surv Ophthalmol. 2004; 49:Suppl 1. S5–S11.2. Hylton C, Robin AL. Update on prostaglandin analogs. Curr Opin Ophthalmol. 2003; 14:65–69.3. Vogel R, Strahlman E, Rittenhouse KD. Adverse events associated with commonly used glaucoma drugs. Int Ophthalmol Clin. 1999; 39:107–124.4. Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004; 81:574–577.5. Lee JW, Kim DY, Lee YK. Two cases of deepening of the upper lid sulcus from topical bimatoprost therapy. J Korean Ophthalmol Soc. 2007; 48:332–336.6. Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008; 24:302–307.7. Tappeiner C, Perren B, Iliev ME, et al. Orbital fat atrophy in glaucoma patients treated with topical bimatoprost: can bimatoprost cause enophthalmos? Klin Monbl Augenheilkd. 2008; 225:443–445.8. Yam JC, Yuen NS, Chan CW. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther. 2009; 25:471–472.9. Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol. 2009; 53:176–179.10. Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011; 55:22–27.11. Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974; 1:113–116.12. Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980; 255:4745–4750.13. Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994; 14:99–129.14. Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974; 3:127–133.15. Casimir DA, Miller CW, Ntambi JM. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation. 1996; 60:203–210.16. Abramovitz M, Boie Y, Nguyen T, et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994; 269:2632–2636.17. Serrero G, Lepak N. Endocrine and paracrine negative regulators of adipose differentiation. Int J Obes Relat Metab Disord. 1996; 20:Suppl 3. S58–S64.18. Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997; 233:200–202.19. Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem. 1998; 273:1855–1858.20. Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology. 1996; 137:5641–5650.21. Bernlohr DA, Bolanowski MA, Kelly TJ Jr, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985; 260:5563–5567.22. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.23. Green H. Adipose conversion: a program of differentiation. In : Ailhaud G, editor. Obesity: cellular and molecular aspects. Paris: Colloques INSERM;1979. p. 15–24.24. Amri EZ, Dani C, Doglio A, et al. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986; 137:903–910.25. Bernlohr DA, Angus CW, Lane MD, et al. Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984; 81:5468–5472.26. Tontonoz P, Hu E, Graves RA, et al. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994; 8:1224–1234.27. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156.28. Ntambi JM, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000; 130:3122S–3126S.29. Kraft ME, Glaeser H, Mandery K, et al. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010; 51:2504–2511.30. Dukes M, Russell W, Walpole AL. Potent luteolytic agents related to prostaglandin F2alpha. Nature. 1974; 250:330–331.31. Serrero G, Lepak NM, Goodrich SP. Prostaglandin F2 alpha inhibits the differentiation of adipocyte precursors in primary culture. Biochem Biophys Res Commun. 1992; 183:438–442.32. Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003; 19:501–515.33. Sharif NA, Crider JY, Husain S, et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+ mobilization and MAP kinase activation. J Ocul Pharmacol Ther. 2003; 19:437–455.34. Choi HY, Lee JE, Lee JW, et al. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J Ocul Pharmacol Ther. 2012; 28:146–152.35. Lepak NM, Serrero G. Prostaglandin F2 alpha stimulates transforming growth factor-alpha expression in adipocyte precursors. Endocrinology. 1995; 136:3222–3229.36. Chernick SS, Spooner PM, Garrison MM, Scow RO. Effect of epinephrine and other lipolytic agents on intracellular lipolysis and lipoprotein lipase activity in 3T3-L1 adipocytes. J Lipid Res. 1986; 27:286–294.37. Feingold KR, Doerrler W, Dinarello CA, et al. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology. 1992; 130:10–16.38. Wolfram-Gabel R, Kahn JL. Adipose body of the orbit. Clin Anat. 2002; 15:186–192.39. Bujalska IJ, Durrani OM, Abbott J, et al. Characterisation of 11beta-hydroxysteroid dehydrogenase 1 in human orbital adipose tissue: a comparison with subcutaneous and omental fat. J Endocrinol. 2007; 192:279–288.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Adipocyte Differentiation via MicroRNAs
- Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1
- Transcriptional repression of type I procollagen genes during adipocyte differentiation
- Investigation for the Uses of Topical Ophthalmic Drugs Without Doctor's Prescription
- TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells