Immune Netw.
2015 Dec;15(6):278-290. 10.4110/in.2015.15.6.278.
3,3'-Diindolylmethane Inhibits Flt3L/GM-CSF-induced-bone Marrow-derived CD103+ Dendritic Cell Differentiation Regulating Phosphorylation of STAT3 and STAT5
- Affiliations
-
- 1Department of Biology, Changwon National University, Changwon 51140, Korea. parkjh@changwon.ac.kr
- KMID: 2132702
- DOI: http://doi.org/10.4110/in.2015.15.6.278
Abstract
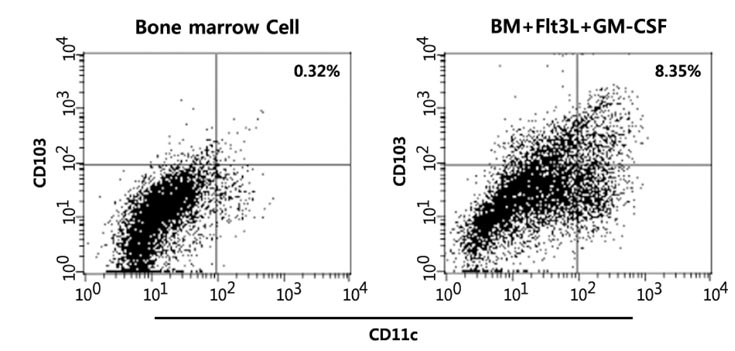
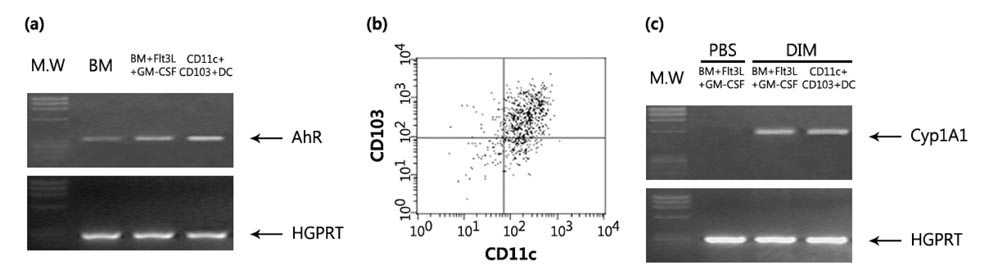
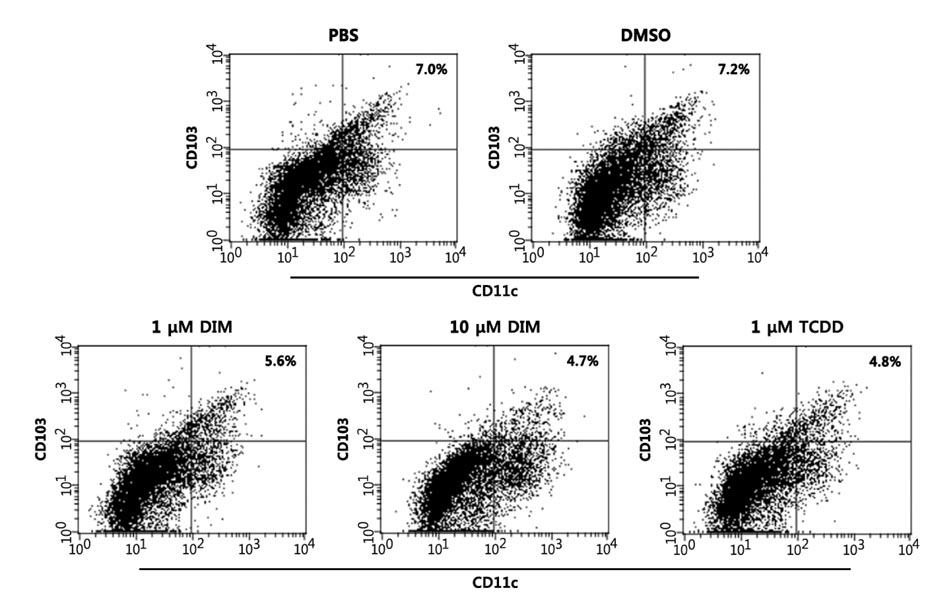
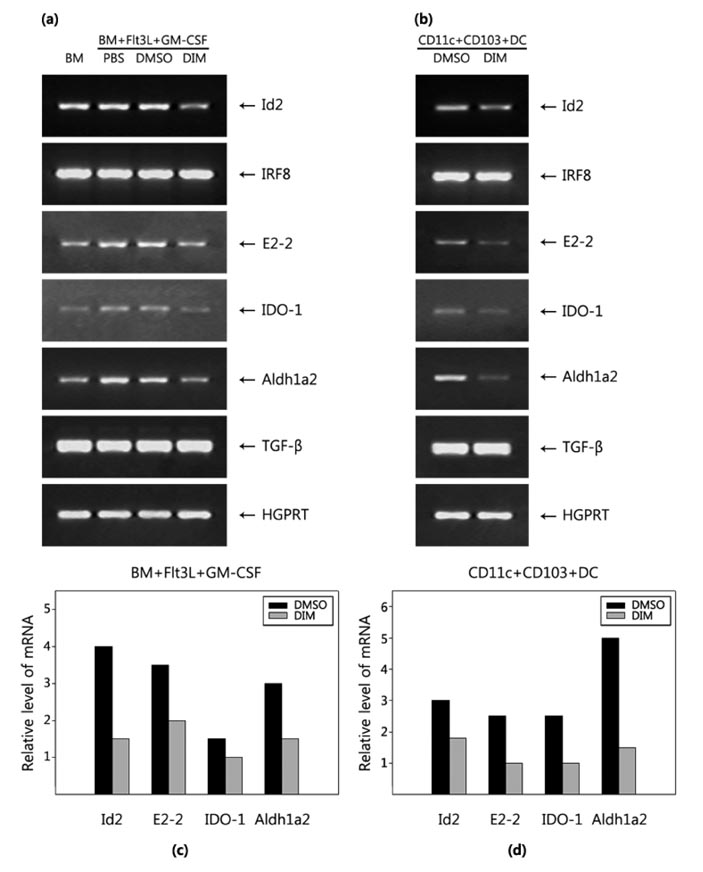
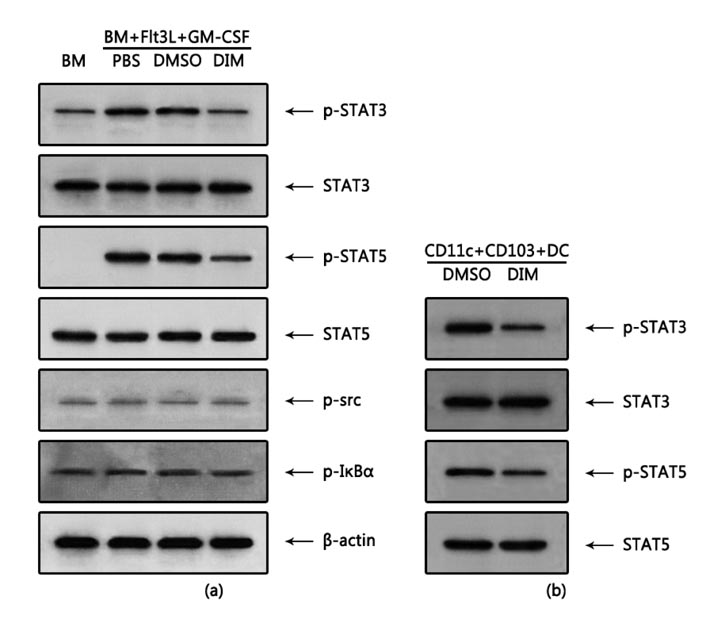
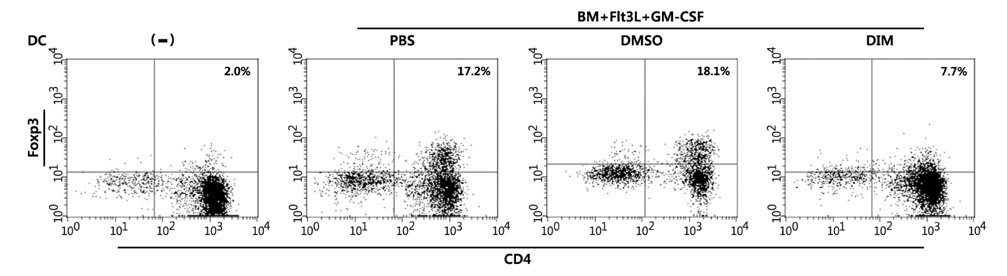
- The intestinal immune system maintains oral tolerance to harmless antigens or nutrients. One mechanism of oral tolerance is mediated by regulatory T cell (Treg)s, of which differentiation is regulated by a subset of dendritic cell (DC)s, primarily CD103+ DCs. The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, plays an important role in regulating immunity. The intestines are exposed to various AhR ligands, including endogenous metabolites and phytochemicals. It was previously reported that AhR activation induced tolerogenic DCs in mice or in cultures of bone marrow-derived DCs. However, given the variety of tolerogenic DCs, which type of tolerogenic DCs is regulated by AhR remains unknown. In this study, we found that AhR ligand 3,3'-diindolylmethane (DIM) inhibited the development of CD103+ DCs from mouse bone marrow cells stimulated with Flt3L and GM-CSF. DIM interfered with phosphorylation of STAT3 and STAT5 inhibiting the expression of genes, including Id2, E2-2, IDO-1, and Aldh1a2, which are associated with DC differentiation and functions. Finally, DIM suppressed the ability of CD103+ DCs to induce Foxp3+ Tregs.
Keyword
MeSH Terms
-
Animals
Bone Marrow Cells
Dendritic Cells*
Granulocyte-Macrophage Colony-Stimulating Factor
Immune System
Intestines
Ligands
Mice
Phosphorylation*
Phytochemicals
Receptors, Aryl Hydrocarbon
Transcription Factors
Granulocyte-Macrophage Colony-Stimulating Factor
Ligands
Receptors, Aryl Hydrocarbon
Transcription Factors
Figure
Cited by 1 articles
-
Aryl Hydrocarbon Receptor Ligands Indoxyl 3-sulfate and Indole-3-carbinol Inhibit FMS-like Tyrosine Kinase 3 Ligand-induced Bone Marrow-derived plasmacytoid Dendritic Cell Differentiation
Won-Bhin Hwang, Da-Jeong Kim, Gap-Soo Oh, Joo-Hung Park
Immune Netw. 2018;18(5):. doi: 10.4110/in.2018.18.e35.
Reference
-
1. Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992; 89:8185–8189.
Article2. Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990; 265:14648–14653.
Article3. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993; 3:213–230.
Article4. Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD: experimental, mechanistic, and epidemiologic evidence. Annu Rev Pharmacol Toxicol. 1994; 34:343–372.
Article5. Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008; 21:102–116.
Article6. Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007; 581:3608–3615.
Article7. Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta. 2013; 1836:197–210.
Article8. Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995; 204:133–143.
Article9. Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004; 36:189–204.
Article10. Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009; 77:713–722.
Article11. Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013; 65:1148–1161.12. Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003; 252:92–98.
Article13. Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008; 205:2139–2149.
Article14. Coombes JL, Siddiqui KR, rancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007; 204:1757–1764.
Article15. Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006; 176:6752–6761.
Article16. Perdew GH, Babbs CF. Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer. 1991; 16:209–218.
Article17. Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998; 37:11508–11515.
Article18. Yaktine AL, Harrison GG, Lawrence RS. Reducing exposure to dioxins and related compounds through foods in the next generation. Nutr Rev. 2006; 64:403–409.
Article19. Aires A, Mota VR, Saavedra MJ, Rosa EA, Bennett RN. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J Appl Microbiol. 2009; 106:2086–2095.
Article20. Celloto VR, Oliveira AJ, Goncalves JE, Watanabe CS, Matioli G, Goncalves RA. Biosynthesis of indole-3-acetic acid by new Klebsiella oxytoca free and immobilized cells on inorganic matrices. ScientificWorldJournal. 2012; 495970.21. Stange J, Veldhoen M. The aryl hydrocarbon receptor in innate T cell immunity. Semin Immunopathol. 2013; 35:645–655.
Article22. Simones T, Shepherd DM. Consequences of AhR activation in steady-state dendritic cells. Toxicol Sci. 2011; 119:293–307.
Article23. Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010; 107:19961–19966.
Article24. Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008; 112:1214–1222.
Article25. Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010; 107:20768–20773.
Article26. Liu J, Cao X. Regulatory dendritic cells in autoimmunity: A comprehensive review. J Autoimmun. 2015; 63:1–12.
Article27. Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007; 8:1380–1389.
Article28. Kinoshita H, Abe J, Akadegawa K, Yurino H, Uchida T, Ikeda S, Matsushima K, Ishikawa S. Breakdown of mucosal immunity in gut by 2,3,7,8-tetraclorodibenzo-p-dioxin (TCDD). Environ Health Prev Med. 2006; 11:256–263.
Article29. Chmill S, Kadow S, Winter M, Weighardt H, Esser C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs stable establishment of oral tolerance in mice. Toxicol Sci. 2010; 118:98–107.
Article30. Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991; 88:9543–9547.
Article31. Jackson JT, Hu Y, Liu R, Masson F, D'Amico A, Carotta S, Xin A, Camilleri MJ, Mount AM, Kallies A, Wu L, Smyth GK, Nutt SL, Belz GT. Id2 expression delineates differential checkpoints in the genetic program of CD8alpha+ and CD103+ dendritic cell lineages. EMBO J. 2011; 30:2690–2704.
Article32. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007; 204:1775–1785.
Article33. Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di PT, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007; 219:118–142.
Article34. Frericks M, Meissner M, Esser C. Microarray analysis of the AHR system: tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol. 2007; 220:320–332.
Article35. Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009; 182:6709–6717.
Article36. Miniero R, De FE, Ferri F, di DA. An overview of TCDD half-life in mammals and its correlation to body weight. Chemosphere. 2001; 43:839–844.
Article37. Bradfield CA, Bjeldanes LF. Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987; 21:311–323.
Article38. Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010; 238:76–92.
Article39. Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011; 32:412–419.40. Li HS, Yang CY, Nallaparaju KC, Zhang H, Liu YJ, Goldrath AW, Watowich SS. The signal transducers STAT5 and STAT3 control expression of Id2 and E2-2 during dendritic cell development. Blood. 2012; 120:4363–4373.
Article41. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–3393.
Article42. Hwang SJ, Hwang YJ, Yun MO, Kim JH, Oh GS, Park JH. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol Lett. 2013; 220:109–117.
Article43. Murante FG, Gasiewicz TA. Hemopoietic progenitor cells are sensitive targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J mice. Toxicol Sci. 2000; 54:374–383.
Article44. Sakai R, Kajiume T, Inoue H, Kanno R, Miyazaki M, Ninomiya Y, Kanno M. TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cells. Toxicol Sci. 2003; 72:84–91.
Article45. Gasiewicz TA, Singh KP, Bennett JA. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci. 2014; 1310:44–50.
Article46. Lee JA, Hwang JA, Sung HN, Jeon CH, Gill BC, Youn HJ, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2007; 173:31–40.
Article47. Hwang JA, Lee JA, Cheong SW, Youn HJ, Park JH. Benzo(a)pyrene inhibits growth and functional differentiation of mouse bone marrow-derived dendritic cells. Downregulation of RelB and eIF3 p170 by benzo(a)pyrene. Toxicol Lett. 2007; 169:82–90.
Article48. Beamer CA, Seaver BP, Shepherd DM. Aryl hydrocarbon receptor (AhR) regulates silica-induced inflammation but not fibrosis. Toxicol Sci. 2012; 126:554–568.
Article49. Stobbe-Maicherski N, Wolff S, Wolff C, Abel J, Sydlik U, Frauenstein K, Haarmann-Stemmann T. The interleukin-6-type cytokine oncostatin M induces aryl hydrocarbon receptor expression in a STAT3-dependent manner in human HepG2 hepatoma cells. FEBS J. 2013; 280:6681–6690.
Article50. Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008; 105:9721–9726.
Article51. Jeong KT, Hwang SJ, Oh GS, Park JH. FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma. Int Immunopharmacol. 2012; 13:377–385.
Article52. Hwang YJ, Yun MO, Jeong KT, Park JH. Uremic toxin indoxyl 3-sulfate regulates the differentiation of Th2 but not of Th1 cells to lessen allergic asthma. Toxicol Lett. 2014; 225:130–138.
Article53. Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999; 274:17209–17218.
Article54. Kazansky AV, Kabotyanski EB, Wyszomierski SL, Mancini MA, Rosen JM. Differential effects of prolactin and src/abl kinases on the nuclear translocation of STAT5B and STAT5A. J Biol Chem. 1999; 274:22484–22492.
Article55. Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol. 1996; 52:1599–1612.
Article56. Dong B, Cheng W, Li W, Zheng J, Wu D, Matsumura F, Vogel CF. FRET analysis of protein tyrosine kinase c-Src activation mediated via aryl hydrocarbon receptor. Biochim Biophys Acta. 2011; 1810:427–431.
Article57. Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, la Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014; 511:184–190.
Article58. Superti-Furga G, Fumagalli S, Koegl M, Courtneidge SA, Draetta G. Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J. 1993; 12:2625–2634.
Article59. Rey-Barroso J, Colo GP, varez-Barrientos A, Redondo-Munoz J, Carvajal-Gonzalez JM, Mulero-Navarro S, Garcia-Pardo A, Teixido J, Fernandez-Salguero PM. The dioxin receptor controls beta1 integrin activation in fibroblasts through a Cbp-Csk-Src pathway. Cell Signal. 2013; 25:848–859.
Article60. Roskoski R Jr. Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004; 324:1155–1164.
Article61. Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, Zenke M. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003; 4:380–386.
Article62. Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009; 206:3115–3130.63. Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol. 2008; 38:2389–2400.
Article64. Driggers PH, Ennist DL, Gleason SL, Mak WH, Marks MS, Levi BZ, Flanagan JR, Appella E, Ozato K. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1990; 87:3743–3747.
Article65. Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013; 121:1839–1849.
Article66. Sasaki H, Kurotaki D, Osato N, Sato H, Sasaki I, Koizumi S, Wang H, Kaneda C, Nishiyama A, Kaisho T, Aburatani H, Morse HC III, Ozato K, Tamura T. Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood. 2015; 125:358–369.
Article67. Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005; 174:2573–2581.
Article68. Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008; 135:37–48.
Article69. Contador-Troca M, varez-Barrientos A, Merino JM, Morales-Hernandez A, Rodriguez MI, Rey-Barroso J, Barrasa E, Cerezo-Guisado MI, Catalina-Fernandez I, Saenz-Santamaria J, Oliver FJ, Fernandez-Salguero PM. Dioxin receptor regulates aldehyde dehydrogenase to block melanoma tumorigenesis and metastasis. Mol Cancer. 2015; 14:148.
Article70. Kazantseva MG, Highton J, Stamp LK, Hessian PA. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res Ther. 2012; 14:R208.
Article71. Noh KT, Son KH, Jung ID, Kang TH, Choi CH, Park YM. Glycogen Synthase Kinase-3beta (GSK-3beta) Inhibition Enhances Dendritic Cell-based Cancer Vaccine Potency via Suppression of Interferon-gamma-induced Indoleamine 2,3-Dioxygenase Expression. J Biol Chem. 2015; 290:12394–12402.
Article72. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008; 453:65–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aryl Hydrocarbon Receptor Ligands Indoxyl 3-sulfate and Indole-3-carbinol Inhibit FMS-like Tyrosine Kinase 3 Ligand-induced Bone Marrow-derived plasmacytoid Dendritic Cell Differentiation
- The Clinical Significance of STAT3 and STAT5 Activation in Renal Cell Carcinoma
- Serum amyloid A inhibits dendritic cell differentiation by suppressing GM-CSF receptor expression and signaling
- Regulation of Osteoclast Differentiation: Identification of osteoclast and macrophage fusion protein; DC-STAMP
- Extended Culture of Bone Marrow with Granulocyte Macrophage-Colony Stimulating Factor Generates Immunosuppressive Cells