Korean J Hepatobiliary Pancreat Surg.
2015 Nov;19(4):139-148. 10.14701/kjhbps.2015.19.4.139.
In vitro immune cell monitoring as a guide for long-term immunosuppression in adult liver transplant recipients
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 2Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2130965
- DOI: http://doi.org/10.14701/kjhbps.2015.19.4.139
Abstract
- BACKGROUNDS/AIMS
We evaluated the clinical usability of immune cell monitoring in adult liver transplantation (LT) recipients.
METHODS
This study was composed of two parts as using calcineurin phosphatase (CNP) activity assay and ImmuKnow assay independently as in vitro monitoring tools of immune cell function in adult LT recipients.
RESULTS
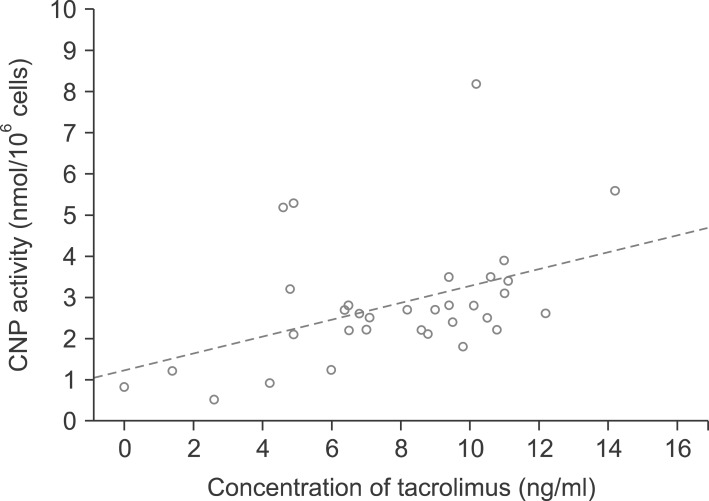
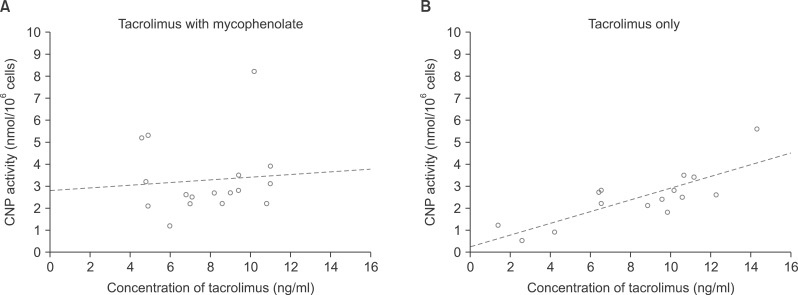
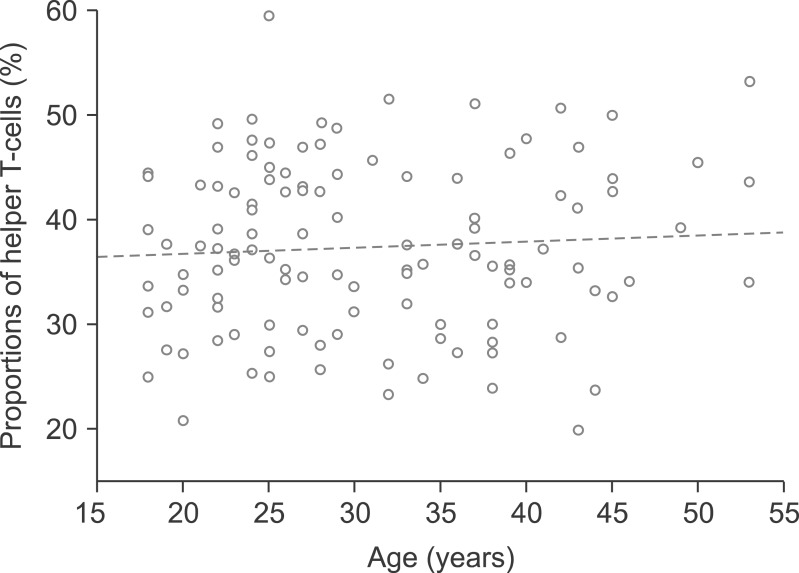
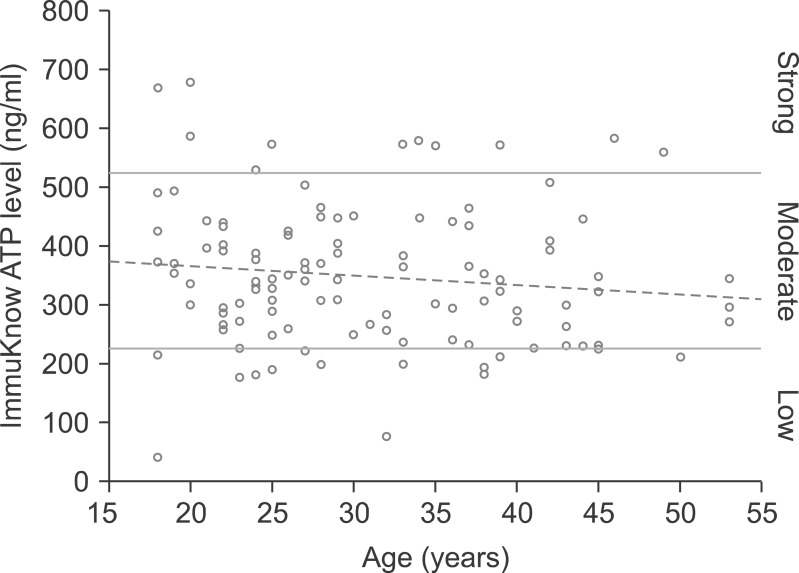
There was a rough correlation between CNP activity and tacrolimus concentration in 33 patients. This association was evident in patients who were only administered tacrolimus, but disappeared after the co-administration of mycophenolate. In 118 healthy individuals, the mean proportion of helper T-cells was 37.4+/-8.1%. According to ImmuKnow assay, their immune responses were strong in 12 patients (10.2%), moderate in 92 patients (78.0%), and low in 14 patients (11.9%). In 85 patients waiting for LT, there was a rough correlation between the ImmuKnow ATP level and age. Their immune responses were strong in 0 patients (0%), moderate in 8 patients (9.4%), and low in 77 patients (90.6%). There was a difference in the ImmuKnow ATP levels between healthy individuals and patients with liver disease. In 137 LT recipients, there was no correlation between the ImmuKnow ATP levels and tacrolimus concentration. This trend did not change after grouping the patients according to co-administration with mycophenolate. Eight recipients experienced acute rejection, but none showed strong immune response.
CONCLUSIONS
We think that both CNP activity assay and ImmuKnow assay are too limited to objectively determine the level of immunosuppression. Further studies should be performed to identify other methods for immune function monitoring.
MeSH Terms
Figure
Reference
-
1. Fukatsu S, Yano I, Igarashi T, Hashida T, Takayanagi K, Saito H, et al. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol. 2001; 57:479–484. PMID: 11699612.2. Fukudo M, Yano I, Fukatsu S, Saito H, Uemoto S, Kiuchi T, et al. Forecasting of blood tacrolimus concentrations based on the Bayesian method in adult patients receiving living-donor liver transplantation. Clin Pharmacokinet. 2003; 42:1161–1178. PMID: 14531726.
Article3. Fukudo M, Yano I, Masuda S, Fukatsu S, Katsura T, Ogura Y, et al. Pharmacodynamic analysis of tacrolimus and cyclosporine in living-donor liver transplant patients. Clin Pharmacol Ther. 2005; 78:168–181. PMID: 16084851.
Article4. Liu XQ, Hu ZQ, Pei YF, Tao R. Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects. Hepatobiliary Pancreat Dis Int. 2013; 12:12–33. PMID: 23392795.
Article5. de la Garza RG, Sarobe P, Merino J, Lasarte JJ, D'Avola D, Belsue V, et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transpl. 2013; 19:937–944. PMID: 23784747.
Article6. Londoño MC, Rimola A, O'Grady J, Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J Hepatol. 2013; 59:872–879. PMID: 23578883.
Article7. Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013; 58:1824–1835. PMID: 23532679.
Article8. Bohne F, Martínez-Llordella M, Lozano JJ, Miquel R, Benítez C, Londoño MC, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012; 122:368–382. PMID: 22156196.
Article9. Fukudo M, Yano I, Masuda S, Okuda M, Inui K. Distinct inhibitory effects of tacrolimus and cyclosporin a on calcineurin phosphatase activity. J Pharmacol Exp Ther. 2005; 312:816–825. PMID: 15383634.
Article10. Fukudo M, Yano I, Masuda S, Katsura T, Ogura Y, Oike F, et al. Cyclosporine exposure and calcineurin phosphatase activity in living-donor liver transplant patients: twice daily vs. once daily dosing. Liver Transpl. 2006; 12:292–300. PMID: 16447186.
Article11. Kung L, Halloran PF. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplantation. 2000; 70:327–335. PMID: 10933159.12. Hwang S, Kim KH, Song GW, Yu YD, Park GC, Kim KW, et al. Peritransplant monitoring of immune cell function in adult living donor liver transplantation. Transplant Proc. 2010; 42:2567–2571. PMID: 20832545.
Article13. van Rossum HH, Romijn FP, Sellar KJ, Smit NP, van der Boog PJ, de Fijter JW, et al. Variation in leukocyte subset concentrations affects calcineurin activity measurement: implications for pharmacodynamic monitoring strategies. Clin Chem. 2008; 54:517–524. PMID: 18218723.
Article14. Blanchet B, Duvoux C, Costentin CE, Barrault C, Ghaleh B, Salvat A, et al. Pharmacokinetic-pharmacodynamic assessment of tacrolimus in liver-transplant recipients during the early post-transplantation period. Ther Drug Monit. 2008; 30:412–418. PMID: 18641556.
Article15. Yano I. Pharmacodynamic monitoring of calcineurin phosphatase activity in transplant patients treated with calcineurin inhibitors. Drug Metab Pharmacokinet. 2008; 23:150–157. PMID: 18574318.
Article16. Cremers S, Schoemaker R, Scholten E, den Hartigh J, König-Quartel J, van Kan E, et al. Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol. 2005; 60:249–256. PMID: 16120063.
Article17. Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M, et al. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004; 10:492–502. PMID: 15048791.
Article18. Pisupati J, Jain A, Burckart G, Hamad I, Zuckerman S, Fung J, et al. Intraindividual and interindividual variations in the pharmacokinetics of mycophenolic acid in liver transplant patients. J Clin Pharmacol. 2005; 45:34–41. PMID: 15601803.
Article19. Brown NW, Aw MM, Mieli-Vergani G, Dhawan A, Tredger JM. Mycophenolic acid and mycophenolic acid glucuronide pharmacokinetics in pediatric liver transplant recipients: effect of cyclosporine and tacrolimus comedication. Ther Drug Monit. 2002; 24:598–606. PMID: 12352931.
Article20. Hesselink DA, van Hest RM, Mathot RA, Bonthuis F, Weimar W, de Bruin RW, et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant. 2005; 5:987–994. PMID: 15816878.
Article21. Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003; 17:77–88. PMID: 12709071.
Article22. Rodrigo E, López-Hoyos M, Corral M, Fábrega E, Fernández-Fresnedo G, San Segundo D, et al. ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012; 18:1245–1253. PMID: 22740321.
Article23. Ravaioli M, Neri F, Lazzarotto T, Bertuzzo VR, Di Gioia P, Stacchini G, et al. Immunosuppression Modifications Based on an Immune Response Assay: Results of a Randomized, Controlled Trial. Transplantation. 2015; 99:1625–1632. PMID: 25757214.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunizations in adult liver transplant candidates and recipients
- Squamous Cell Carcinomain Renal Transplant Recipient
- Clinical Immune Tolerance in Liver Transplantation: Present and Future
- Advancing immunosuppression in liver transplantation: the role of regulatory T cells in immune modulation and graft tolerance
- Pediatric Liver Transplantation