Pediatric Liver Transplantation
- Affiliations
-
- 1Division of Pediatric Surgery, Deptartment of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sukkoo.lee@samsung.com
- KMID: 1427980
- DOI: http://doi.org/10.13029/jkaps.2013.19.1.14
Abstract
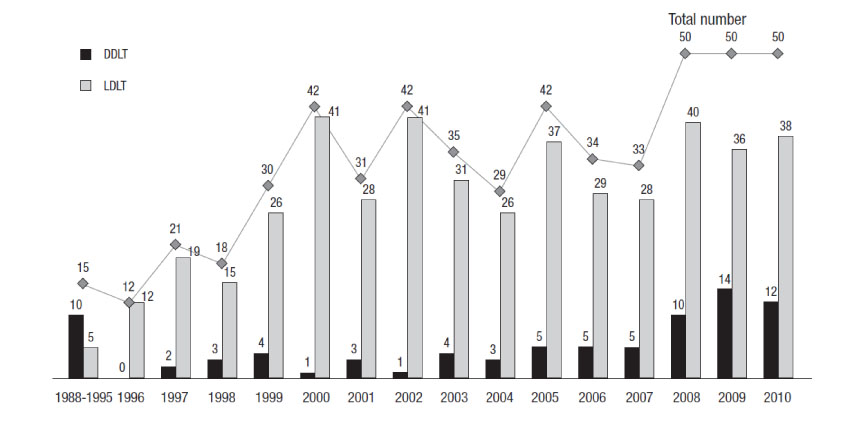
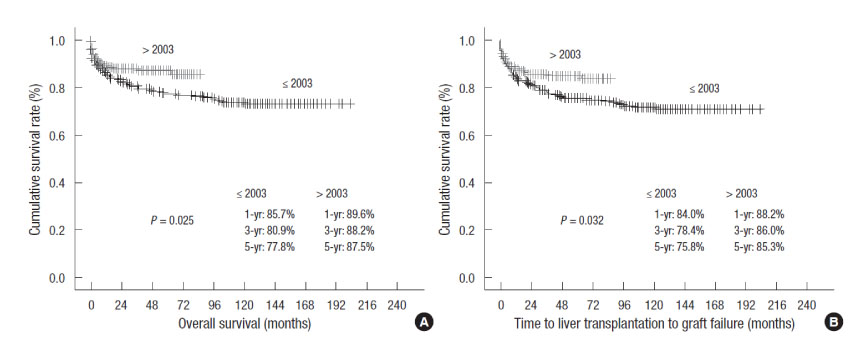
- Pediatric liver transplantation has evolved into a definite and effective therapeutic modality for various liver diseases in the pediatric patient. During the last 25 years, liver transplant outcomes in Korea have reached international standards and Korea has become the leader in living-donor liver transplantation. This review will present the cumulative outcomes of pediatric liver transplantation performed in Korea and will focus on other issues of interest involving pediatric liver transplant recipients, especially in the field of immunosuppression and post-transplant lymphoproliferative disease.
Keyword
Figure
Cited by 5 articles
-
Pediatric liver transplantation in Korea: long-term outcomes and allocations
Sanghoon Lee, Suk-Koo Lee
Korean J Transplant. 2019;33(1):1-5. doi: 10.4285/jkstn.2019.33.1.1.Pediatric liver transplantation with hyperreduced left lateral segment graft
Jung-Man Namgoong, Shin Hwang, Gi-Won Song, Dae-Yeon Kim, Tae-Yong Ha, Dong-Hwan Jung, Gil-Chun Park, Chul-Soo Ahn, Kyung Mo Kim, Seak Hee Oh, Hyunhee Kwon, Yong Jae Kwon
Ann Hepatobiliary Pancreat Surg. 2020;24(4):503-512. doi: 10.14701/ahbps.2020.24.4.503.Prognosis of Split Liver Transplantation Compared with Whole Liver Transplantation in Adult Patients: Single-center Results under the Korean MELD Score-based Allocation Policy
Gil-Chun Park, Shin Hwang, Gi-Won Song, Dong-Hwan Jung, Tae-Yong Ha, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Young-In Yoon, Woo-Hyoung Kang, Hwui-Dong Cho, Jin Uk Choi, Minjae Kim, Byeong-Gon Na, Sang Hoon Kim, Sung-Gyu Lee
J Korean Med Sci. 2020;35(37):e304. doi: 10.3346/jkms.2020.35.e304.Pediatric liver transplantation in Korea: long-term outcomes and allocations
Sanghoon Lee, Suk-Koo Lee
J Korean Soc Transplant. 2019;33(1):1-5. doi: 10.4285/jkstn.2019.33.1.1.Twenty-year longitudinal follow-up after liver transplantation: a single-center experience with 251 consecutive patients
Minjae Kim, Shin Hwang, Chul-Soo Ahn, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Ki-Hun Kim, Jung-Man Namgoong, Woo-Hyoung Kang, Young-In Yoon, Hwui-Dong Cho, Byeong-Gon Na, Sang Hoon Kim, Sung-Gyu Lee
Korean J Transplant. 2022;36(1):45-53. doi: 10.4285/kjt.21.0031.
Reference
-
1. Starzl TE, Koep LJ, Schroter GP, Halgrimson CG, Porter KA, Weil R 3rd. Liver replacement for pediatric patients. Pediatrics. 1979; 63:825–829.2. Kim MJ, Choe YH. Indication of pediatric liver transplantation. J Korean Soc Transplant. 2011; 25:151–154.3. Ryckman FC, Alonso MH, Bucuvalas JC, Balistreri WF. Biliary atresia-surgical management and treatment options as they relate to outcome. Liver Transpl Surg. 1998; 4:5 Suppl 1. S24–S33.4. Arnon R, Kerkar N, Davis MK, Anand R, Yin W, Gonzalez-Peralta RP. Liver transplantation in children with metabolic diseases: the studies of pediatric liver transplantation experience. Pediatr Transplant. 2010; 14:796–805.5. Kim ST, Park YH, Lee KU, Yoon YK, Kim SW, Yang HK, Kim WK, Park KW. An experience of liver transplantation in Korea. J Korean Soc Transplant. 1988; 2:27–36.6. Kim JM, Kim KM, Yi NJ, Choe YH, Kim MS, Suh KS, Kim SI, Lee SK, Lee SG. Pediatric liver transplantation outcomes in Korea. J Korean Med Sci. 2013; 28:42–47.7. Busuttil RW, Klintmalm GB. General criteria for pediatric transplantation. Transplantation of the liver. second edition. Philadelphia, PA: Elsevier Saunders;2005. p. 288–291. chap 21.8. Kim JM, Lee SK, Kwon CH, Joh JW, Choe YH, Park CK. Hepatocellular carcinoma in an infant with biliary atresia younger than 1 year. J Pediatr Surg. 2012; 47:819–821.9. Choi Y, Lee KW, Hong G, Kim H, Park MS, Suh S, Yoo T, Lee HW, Yi NJ, Suh KS. Status and Current Problems in the Allocation System for Pediatric Liver Transplantation in Korea. J Korean Soc Transplant. 2012; 26:196–201.10. United Nework for Organ Sharing (UNOS). Organ Distribution: Allocation of Livers [Internet]. Richmond, VI: UNOS;2011. Available from: http://optn.transplant.hrsa.gov.11. Nalesnik MA. The diverse pathology of post-transplant lymphoproliferative disorders: the importance of a standardized approach. Transpl Infect Dis. 2001; 3:88–96.12. Preiksaitis JK, Keay S. Diagnosis and management of posttransplant lymphoproliferative disorder in solid-organ transplant recipients. Clin Infect Dis. 2001; 33:Suppl 1. S38–S46.13. Preiksaitis JK, Cockfield SM. Bowden RA, Ljungman P, Paya CV, editors. Epstein-Barr virus and lymphoproliferative disease after hemopoietic stem cell or solid organ transplantation. Transplant Infections. 2nd edn. Lippincott: Williams, and Wilkins;2003. p. 326–349.14. Green M, Reyes J, Webber S, Rowe D. The role of antiviral and immunoglobulin therapy in the prevention of Epstein-Barr virus infection and post-transplant lymphoproliferative disease following solid organ transplantation. Transpl Infect Dis. 2001; 3:97–103.15. Epstein-Barr virus and lymphoproliferative disorders after transplantation. Am J Transplant. 2004; 4:Suppl.10. 59–65.16. Choe YH, Lee SK, Seo JM, Joh JW, Kim SJ, Lee KW, Park JH, Ko YH, Kwon KY. Posttransplant lymphoproliferative disorder in pediatric liver transplantation: Samsung Medical Center experience. Korean J Pediatr Gastroenterol Nutr. 2003; 6:39–46.17. Heo JS, Park JW, Lee KW, Lee SK, Joh JW, Kim SJ, Lee HH, Lee DS, Choi SH, Seo JM, Choe YH. Posttrans-plantation lymphoproliferative disorder in pediatric liver transplantation. Transplant Proc. 2004; 36:2307–2308.18. Lee JH, Ko JS, Seo JK, Yi NJ, Suh KS, Lee KU, Kang GH. Posttransplant lymphoproliferative disorder after liver transplantation in pediatric patients: Report from a single-center over 21 years. Korean J Pediatr Gastroenterol Nutr. 2009; 12:199–206.19. Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006; 6:1774–1780.20. Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001; 72:449–454.21. Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012; 307:283–293.22. Lee JH, Lee SK, Lee HJ, Seo JM, Joh JW, Kim SJ, Kwon CH, Choe YH. Withdrawal of immunosuppression in pediatric liver transplant recipients in korea. Yonsei Med J. 2009; 50:784–788.23. Sebagh M, Rifai K, Féray C, Yilmaz F, Falissard B, Roche B. All liver recipients benefit from the protocol 10-year liver biopsies. Hepatology. 2003; 37:1293–1301.24. Koshiba T, Li Y, Takemura M, Wu Y, Sakaquchi S, Minato N. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007; 17:94–97.25. Ohe H, Li Y, Nafady-Hego H, Kayo W, Sakaguchi S, Wood K, Calne R, Uemoto S, Koshiba T. Minimal but essential doses of immunosuppression: a more realistic approach to improve long-term outcomes for pediatric living-donor liver transplantation. Transplantation. 2011; 91:808–810.26. Holt RI, Baker AJ, Jones JS, Miell JP. The insulin-like growth factor and binding protein axis in children with end-stage liver disease before and after orthotopic liver transplantation. Pediatr Transplant. 1998; 2:76–84.27. Bartosh SM, Thomas SE, Sutton MM, Brady LM, Whitington PF. Linear growth after pediatric liver transplantation. J Pediatr. 1999; 135:624–631.28. Park SJ, Rim SH, Kim KM, Lee JH, Choi BH, Lee SY, Chang SH, Lee YH, Lee SG. Long-term growth of pediatric patients following living-donor liver transplantation. J Korean Med Sci. 2005; 20:835–840.29. Renz JF, de Roos M, Rosenthal P, Mudge C, Bacchetti P, Watson J, Roberts JP, Ascher NL, Emond JC. Posttrans-plantation growth in pediatric liver recipients. Liver Transpl. 2001; 7:1040–1055.