J Korean Med Sci.
2015 Feb;30(2):167-172. 10.3346/jkms.2015.30.2.167.
Drug-induced Hepatotoxicity of Anti-tuberculosis Drugs and Their Serum Levels
- Affiliations
-
- 1Department of Internal Medicine, National Medical Center, Seoul, Korea.
- 2Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jhlee7@snubh.org
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2129647
- DOI: http://doi.org/10.3346/jkms.2015.30.2.167
Abstract
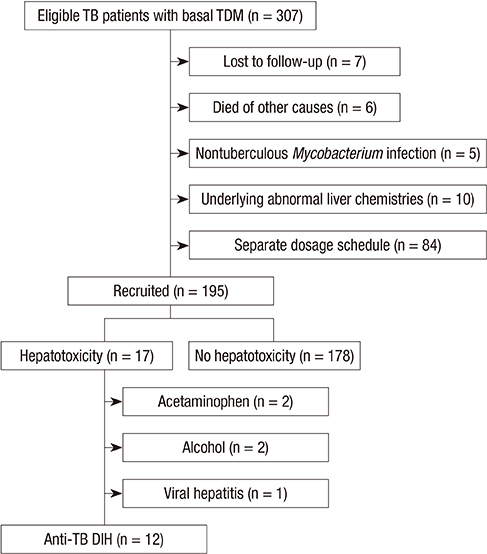
- The correlation between serum anti-tuberculosis (TB) drug levels and the drug-induced hepatotoxicity (DIH) remains unclear. The purpose of this study was to investigate whether anti-TB DIH is associated with basal serum drug levels. Serum peak levels of isoniazid (INH), rifampicin (RMP), pyrazinamide (PZA), and ethambutol (EMB) were analyzed in blood samples 2 hr after the administration of anti-TB medication. Anti-TB DIH and mild liver function test abnormality were diagnosed on the basis of laboratory and clinical criteria. Serum anti-TB drug levels and other clinical factors were compared between the hepatotoxicity and non-hepatotoxicity groups. A total of 195 TB patients were included in the study, and the data were analyzed retrospectively. Seventeen (8.7%) of the 195 patients showed hepatotoxicity, and the mean aspartate aminotransferase/alanine aminotransferase levels in the hepatotoxicity group were 249/249 IU/L, respectively. Among the 17 patients with hepatotoxicity, 12 showed anti-TB DIH. Ten patients showed PZA-related hepatotoxicity and 2 showed INH- or RMP-related hepatotoxicity. However, intergroup differences in the serum levels of the 4 anti-TB drugs were not statistically significant. Basal serum drug concentration was not associated with the risk anti-TB DIH in patients being treated with the currently recommended doses of first-line anti-TB treatment drugs.
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Alanine Transaminase/blood
Antitubercular Agents/adverse effects/*blood/therapeutic use
Aspartate Aminotransferases/blood
Drug-Induced Liver Injury/*blood
Ethambutol/adverse effects/blood/therapeutic use
Female
Humans
Isoniazid/adverse effects/blood/therapeutic use
Liver/*pathology
Liver Function Tests
Male
Middle Aged
Pyrazinamide/adverse effects/blood/therapeutic use
Retrospective Studies
Rifampin/adverse effects/blood/therapeutic use
Tuberculosis, Pulmonary/drug therapy
Young Adult
Antitubercular Agents
Aspartate Aminotransferases
Alanine Transaminase
Ethambutol
Isoniazid
Pyrazinamide
Rifampin
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2012. Geneva: World Health Organization;2012.2. World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva: World Health Organization;2010.3. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662.4. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003; 167:1472–1477.5. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008; 23:192–202.6. Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006; 174:935–952.7. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002; 62:2169–2183.8. Satyaraddi A, Velpandian T, Sharma SK, Vishnubhatla S, Sharma A, Sirohiwal A, Makharia GK, Sinha S, Biswas A, Singh S. Correlation of plasma anti-tuberculosis drug levels with subsequent development of hepatotoxicity. Int J Tuberc Lung Dis. 2014; 18:188–195.9. Wang PY, Xie SY, Hao Q, Zhang C, Jiang BF. NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis. Int J Tuberc Lung Dis. 2012; 16:589–595.10. Donald PR, Parkin DP, Seifart HI, Schaaf HS, van Helden PD, Werely CJ, Sirgel FA, Venter A, Maritz JS. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin Pharmacol. 2007; 63:633–639.11. Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. Hepatotoxicity of pyrazinamide: cohort and case-control analyses. Am J Respir Crit Care Med. 2008; 177:1391–1396.12. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9:2026–2030.13. Durand F, Bernuau J, Pessayre D, Samuel D, Belaiche J, Degott C, Bismuth H, Belghiti J, Erlinger S, Rueff B, et al. Deleterious influence of pyrazinamide on the outcome of patients with fulminant or subfulminant liver failure during antituberculous treatment including isoniazid. Hepatology. 1995; 21:929–932.14. Teleman MD, Chee CB, Earnest A, Wang YT. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int J Tuberc Lung Dis. 2002; 6:699–705.15. Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. Seoul: Korea Centers for Disease Control and Prevention;2011.16. Um SW, Lee SW, Kwon SY, Yoon HI, Park KU, Song J, Lee CT, Lee JH. Low serum concentrations of anti-tuberculosis drugs and determinants of their serum levels. Int J Tuberc Lung Dis. 2007; 11:972–978.17. Song SH, Jun SH, Park KU, Yoon Y, Lee JH, Kim JQ, Song J. Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007; 21:1331–1338.18. Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997; 41:2670–2679.19. Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, Albanese AP, Pitchenik AE. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998; 157:1871–1876.20. Shang P, Xia Y, Liu F, Wang X, Yuan Y, Hu D, Tu D, Chen Y, Deng P, Cheng S, et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011; 6:e21836.21. Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, Chang FY, Lee SD. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology. 2002; 35:883–889.22. Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006; 11:699–707.23. Sharma SK. Antituberculosis drugs and hepatotoxicity. Infect Genet Evol. 2004; 4:167–170.24. Pasipanodya JG, Gumbo T. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob Agents Chemother. 2010; 54:2847–2854.25. Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007; 51:3781–3788.26. Mehta JB, Shantaveerapa H, Byrd RP Jr, Morton SE, Fountain F, Roy TM. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest. 2001; 120:1520–1524.27. Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2007; 51:2546–2551.28. Weiner M, Burman W, Vernon A, Benator D, Peloquin CA, Khan A, Weis S, King B, Shah N, Hodge T. Tuberculosis Trials Consortium. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med. 2003; 167:1341–1347.29. Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest. 1998; 113:1178–1183.30. Heysell SK, Moore JL, Keller SJ, Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis. 2010; 16:1546–1553.31. Lee SW, Chung LS, Huang HH, Chuang TY, Liou YH, Wu LS. NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. Int J Tuberc Lung Dis. 2010; 14:622–626.32. Ray J, Gardiner I, Marriott D. Managing antituberculosis drug therapy by therapeutic drug monitoring of rifampicin and isoniazid. Intern Med J. 2003; 33:229–234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Prophylactic Use of Silymarin on Anti-tuberculosis Drugs Induced Hepatotoxicity
- Clinical Features of Drug-induced Hepatotoxicity During Tuberculosis Treatment
- Concomitant Drug Reaction with Eosinophilia and Systemic Symptom Syndrome from Ethambutol and Autoimmune Hepatitis from Isoniazid
- Factors affecting drug-induced liver injury: antithyroid drugs as instances
- The Influence of Adverse Drug Reactions on First-line Anti-tuberculosis Chemotherapy in the Elderly Patients