Tuberc Respir Dis.
2017 Jul;80(3):265-269. 10.4046/trd.2017.80.3.265.
Effect of Prophylactic Use of Silymarin on Anti-tuberculosis Drugs Induced Hepatotoxicity
- Affiliations
-
- 1Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea. heechung@snu.ac.kr
- 2Department of Medical Statistics, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- KMID: 2385041
- DOI: http://doi.org/10.4046/trd.2017.80.3.265
Abstract
- BACKGROUND
The first line of anti-tuberculosis (TB) drugs are the most effective standard of drugs for TB. However, the use of these drugs is associated with hepatotoxicity. Silymarin has protective effects against hepatotoxicity of anti-TB drugs in animal models. This study aims to investigate the protective effect of silymarin on hepatotoxicity caused by anti-TB drugs.
METHODS
This is a prospective, randomized, double-blind and placebo-controlled study. Patients were eligible if they were 20 years of age or order and started the first-line anti-tuberculosis drugs. Eligible patients were randomized for receiving silymarin or a placebo for the first 4 weeks. The primary outcome was the proportion of patients who showed elevated serum liver enzymes more than 3 times the upper normal limit (UNL) or total bilirubin (TBil) > 2× UNL within the first 8 weeks of anti-TB treatment.
RESULTS
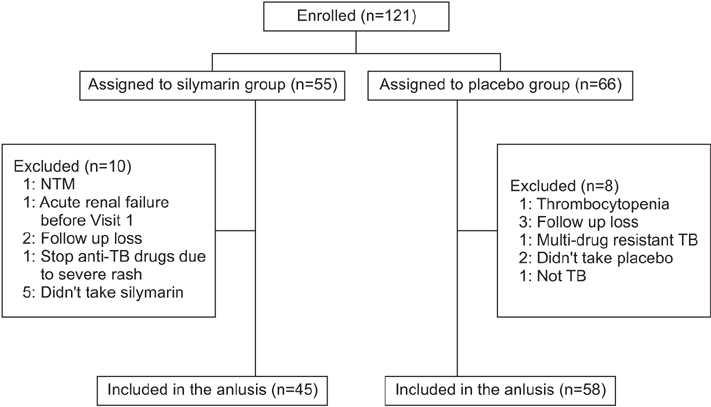
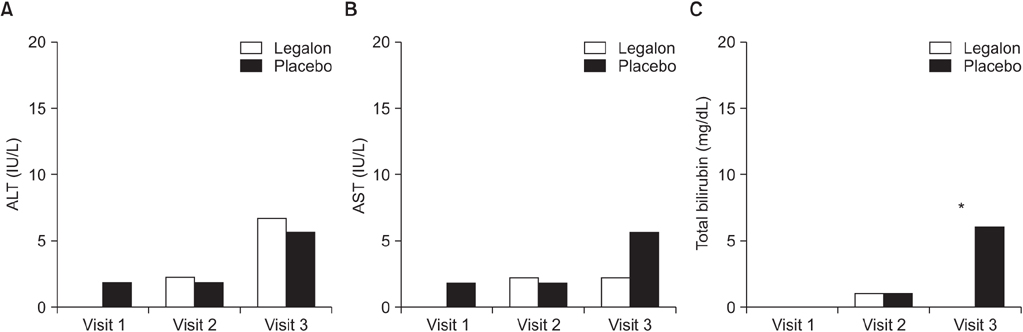
We enrolled a total of 121 patients who silymarin or a placebo to start their anti-TB treatment, for the first 8 weeks. The proportions of elevated serum liver enzymes more than 3 times of UNL at week 2, week 4, and week 8 did not show any significant difference between the silymarin and placebo groups, at 0% versus 3.6% (p>0.999); 4.4% versus 3.6% (p>0.999); and 8.7% versus 10.8% (p=0.630), respectively. However, patients with TBil >2× ULN at week 8 were significantly low in the silymarin group (0% versus 8.7%, p=0.043).
CONCLUSION
Our findings did not show silymarin had any significant preventive effect on the hepatotoxicity of anti-TB drugs.
Keyword
MeSH Terms
Figure
Reference
-
1. Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006; 174:935–952.2. Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw. 2013; 24:114–121.3. Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011; 14:308–317.4. Eminzade S, Uraz F, Izzettin FV. Silymarin protects liver against toxic effects of anti-tuberculosis drugs in experimental animals. Nutr Metab (Lond). 2008; 5:18.5. Jahan S, Khan M, Imran S, Sair M. The hepatoprotective role of Silymarin in isoniazid induced liver damage of rabbits. J Pak Med Assoc. 2015; 65:620–622.6. Liu Q, Garner P, Wang Y, Huang B, Smith H. Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies. BMC Public Health. 2008; 8:365.7. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2014.8. Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012; 107:1380–1387.9. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003; 167:1472–1477.10. Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015; 15:334.11. Agal S, Baijal R, Pramanik S, Patel N, Gupte P, Kamani P, et al. Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol. 2005; 20:1745–1752.12. Saukkonen JJ, Powell K, Jereb JA. Monitoring for tuberculosis drug hepatotoxicity: moving from opinion to evidence. Am J Respir Crit Care Med. 2012; 185:598–599.13. Lee CM, Lee SS, Lee JM, Cho HC, Kim WS, Kim HJ, et al. Early monitoring for detection of antituberculous drug-induced hepatotoxicity. Korean J Intern Med. 2016; 31:65–72.14. Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013; 3:37–49.15. Wu S, Xia Y, Lv X, Tang S, Yang Z, Zhang Y, et al. Preventive use of hepatoprotectors yields limited efficacy on the liver toxicity of anti-tuberculosis agents in a large cohort of Chinese patients. J Gastroenterol Hepatol. 2015; 30:540–545.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Features of Drug-induced Hepatotoxicity During Tuberculosis Treatment
- Anti-oxidant activities of kiwi fruit extract on carbon tetrachloride-induced liver injury in mice
- Drug-induced Hepatotoxicity of Anti-tuberculosis Drugs and Their Serum Levels
- Silymarin Inhibits Cytokine-Stimulated Pancreatic Beta Cells by Blocking the ERK1/2 Pathway
- Inhibition of Wnt Signaling by Silymarin in Human Colorectal Cancer Cells