J Korean Med Sci.
2014 Aug;29(8):1069-1076. 10.3346/jkms.2014.29.8.1069.
Sirolimus Conversion Efficacy for Graft Function Improvement and Histopathology in Renal Recipients with Mild to Moderate Renal Insufficiency
- Affiliations
-
- 1The Research Institute for Transplantation, Severance Hospital, Yonsei University Health System, Seoul, Korea. yukim@yuhs.ac
- 2Department of Transplantation Surgery, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- 3Department of Internal Medicine, Seoul St. Mary Hospital, The Catholic University of Korea, Seoul, Korea.
- 4Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul St. Mary Hospital, The Catholic University of Korea, Seoul, Korea.
- 6Department of Internal Medicine, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- KMID: 2129600
- DOI: http://doi.org/10.3346/jkms.2014.29.8.1069
Abstract
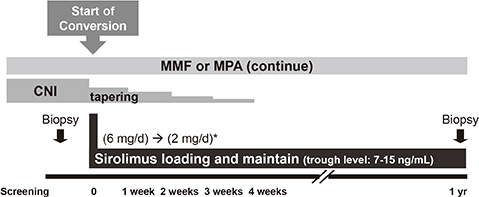
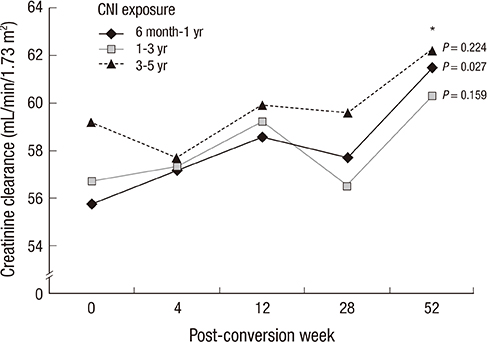
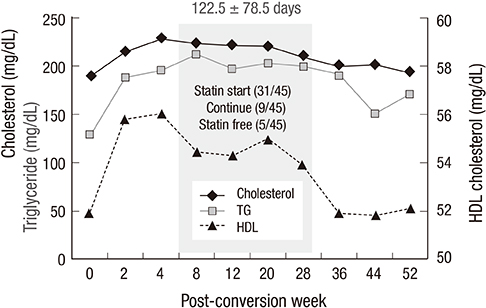
- This study was designed to evaluate whether sirolimus (SRL) conversion effectively improves renal function and histopathology in calcineurin inhibitor (CNI)-treated renal recipients with mild to moderate renal insufficiency. SRL conversion from CNI was performed in patients who underwent kidney transplantation from 6 months to 5 yr prior to screening. Forty-five patients were enrolled. The effect of SRL conversion on graft function was evaluated, and protocol biopsies were performed preconversion and 1 yr after conversion. Overall graft function after SRL conversion gradually improved, and the improvement in renal function was closely associated with the shorter duration of CNI exposure. When we divided the patients by the duration of CNI exposure, the patients with less than 1 yr of CNI exposure demonstrated significant improvement, but patients with a greater than 1 yr CNI exposure did not exhibit significant improvement. In contrast, protocol biopsies demonstrated no significant improvements in the modified "ah" score or other Banff scores after SRL conversion. Furthermore, the duration of CNI treatment prior to SRL conversion was not associated with histological findings 1 yr after SRL conversion. SRL conversion improved graft function in renal recipients with mild to moderate renal insufficiency, but this effect is not accompanied by histological improvement.
MeSH Terms
-
Adult
Calcineurin Inhibitors/*administration & dosage
Drug Synergism
Female
Graft Rejection/*etiology/*prevention & control
Graft Survival/drug effects
Humans
Immunosuppressive Agents
Kidney Transplantation/adverse effects/*methods
Male
Renal Insufficiency/diagnosis/*therapy
Republic of Korea
Severity of Illness Index
Sirolimus/*administration & dosage
Transplantation Tolerance/drug effects
Treatment Outcome
Calcineurin Inhibitors
Immunosuppressive Agents
Sirolimus
Figure
Reference
-
1. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349:2326–2333.2. Chapman JR, Nankivell BJ. Nephrotoxicity of ciclosporin A: short-term gain, long-term pain? Nephrol Dial Transplant. 2006; 21:2060–2063.3. Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney Int. 1996; 50:1089–1100.4. Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, Moulin B, Frouget T, Le Meur Y, Glotz D, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant. 2009; 9:1115–1123.5. Meier-Kriesche HU, Steffen BJ, Chu AH, Loveland JJ, Gordon RD, Morris JA, Kaplan B. Sirolimus with neoral versus mycophenolate mofetil with neoral is associated with decreased renal allograft survival. Am J Transplant. 2004; 4:2058–2066.6. Carvalho C, Coentrão L, Bustorff M, Patrício E, Sampaio S, Santos J, Oliveira G, Pestana M. Conversion from sirolimus to everolimus in kidney transplant recipients receiving a calcineurin-free regimen. Clin Transplant. 2011; 25:E401–E405.7. Flechner SM. Sirolimus in kidney transplantation indications and practical guidelines: de novo sirolimus-based therapy without calcineurin inhibitors. Transplantation. 2009; 87:S1–S6.8. Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, Andrassy J, Reinke P, Pressmar K, Hakenberg O, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation. 2010; 90:175–183.9. Han F, Wu J, Huang H, Zhang X, He Q, Wang Y, Wang S, Wang H, Chen J. Conversion from cyclosporine to sirolimus in chronic renal allograft dysfunction: a 4-year prospective study. Exp Clin Transplant. 2011; 9:42–49.10. Höcker B, Tönshoff B. Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option? Paediatr Drugs. 2011; 13:49–69.11. Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, Wilczek H, Jamieson N, Henriques AC, Paczek L, et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation. 2003; 76:364–370.12. Campistol JM, Cockwell P, Diekmann F, Donati D, Guirado L, Herlenius G, Mousa D, Pratschke J, San Millán JC. Practical recommendations for the early use of m-TOR inhibitors (sirolimus) in renal transplantation. Transpl Int. 2009; 22:681–687.13. Morales JM. Cardiovascular risk profile in patients treated with sirolimus after renal transplantation. Kidney Int Suppl. 2005; (93):S69–S73.14. Schäffer M, Schier R, Napirei M, Michalski S, Traska T, Viebahn R. Sirolimus impairs wound healing. Langenbecks Arch Surg. 2007; 392:297–303.15. Srivastava A, Muruganandham K, Vinodh PB, Singh P, Dubey D, Kapoor R, Kumar A, Sharma RK, Prasad N. Post-renal transplant surgical complications with newer immunosuppressive drugs: mycophenolate mofetil vs. m-TOR inhibitors. Int Urol Nephrol. 2010; 42:279–284.16. Langer RM, Kahan BD. Incidence, therapy, and consequences of lymphocele after sirolimus-cyclosporine-prednisone immunosuppression in renal transplant recipients. Transplantation. 2002; 74:804–808.17. Boratyńska M, Banasik M, Patrzalek D, Szyber P, Klinger M. Sirolimus delays recovery from posttransplant renal failure in kidney graft recipients. Transplant Proc. 2005; 37:839–842.18. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–723.19. Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). Am J Transplant. 2007; 7:518–526.20. Sis B, Dadras F, Khoshjou F, Cockfield S, Mihatsch MJ, Solez K. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006; 6:1444–1450.21. Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, Kalil RN, Pearson TC. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int. 2011; 79:897–907.22. Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Legendre C, Claesson K, Castagneto M, Campistol JM, Hutchison B, et al. Sirolimus-based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. Am J Transplant. 2004; 4:953–961.23. Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, Russ G, Grinyó JM, Stallone G, Hartmann A, et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transpl Int. 2005; 18:22–28.24. Kambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM. A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol. 2007; 2:135–142.25. Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993; 91:2144–2149.26. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009; 4:481–508.27. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003; 349:931–940.28. Lee PC, Lee CY, Hu RH, Lo C, Tsai MK, Lee PH. Intrarenal vascular resistance parameters in kidney transplant patients receiving calcineurin inhibitor-based or sirolimus-based regimens. Nephrol Dial Transplant. 2010; 25:1675–1680.29. Servais A, Meas-Yedid V, Toupance O, Lebranchu Y, Thierry A, Moulin B, Etienne I, Presne C, Hurault de LB, Le Pogamp P, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant. 2009; 9:2552–2560.30. Franco AF, Martini D, Abensur H, Noronha IL. Proteinuria in transplant patients associated with sirolimus. Transplant Proc. 2007; 39:449–452.31. Morelon E, Kreis H. Sirolimus therapy without calcineurin inhibitors: Necker Hospital 8-year experience. Transplant Proc. 2003; 35:52S–57S.32. Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J, Nasou G, Durand D, Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant. 2005; 20:2517–2523.33. Ruiz JC, Campistol JM, Sanchez-Fructuoso A, Mota A, Grinyo JM, Paul J, Castro-Henriques A, Reimao-Pinto J, Garcia J, Morales JM, et al. Early sirolimus use with cyclosporine elimination does not induce progressive proteinuria. Transplant Proc. 2007; 39:2151–2152.34. Watson CJ, Gimson AE, Alexander GJ, Allison ME, Gibbs P, Smith JC, Palmer CR, Bradley JA. A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transpl. 2007; 13:1694–1702.35. Saurina A, Campistol JM, Piera C, Diekmann F, Campos B, Campos N, de las Cuevas X, Oppenheimer F. Conversion from calcineurin inhibitors to sirolimus in chronic allograft dysfunction: changes in glomerular haemodynamics and proteinuria. Nephrol Dial Transplant. 2006; 21:488–493.36. Stallone G, Infante B, Pontrelli P, Gigante M, Montemurno E, Loverre A, Rossini M, Schena FP, Grandaliano G, Gesualdo L. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation. 2011; 91:997–1004.37. Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011; 121:2181–2196.38. Hernández D, Martínez D, Gutiérrez E, López V, Gutiérrez C, García P, Cobelo C, Cabello M, Burgos D, Sola E, et al. Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrologia. 2011; 31:27–34.39. Sayin B, Karakayali H, Colak T, Sevmis S, Pehlivan S, Demirhan B, Haberal M. Conversion to sirolimus for chronic allograft nephropathy and calcineurin inhibitor toxicity and the adverse effects of sirolimus after conversion. Transplant Proc. 2009; 41:2789–2793.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Squamous Cell Carcinoma in Nasal Cavity Treated with Conversion to Sirolimus in a Patient with Kidney Transplantation
- Comparison of Early and Late Conversion of Sirolimus in Experimental Model of Chronic Cyclosporine Nephropathy
- Verruca Eradication Following Conversion to Sirolimus in a Renal Transplant Recipient after Longstanding Cyclosporine A Treatment
- Safety and immunologic benefits of conversion to sirolimus in kidney transplant recipients with long-term exposure to calcineurin inhibitors
- The Relationships between Knowledge of the Kidney, Self-efficacy, and Kidney Function in Pre-dialysis Patients with Chronic Renal Insufficiency