Clin Exp Otorhinolaryngol.
2015 Dec;8(4):312-319. 10.3342/ceo.2015.8.4.312.
An Evaluation of the Protective Effects of Thymoquinone on Amikacin-Induced Ototoxicity in Rats
- Affiliations
-
- 1Department of Otorhinolaryngology, Bezmialem Vakif University, Istanbul, Turkey.
- 2Department of Otorhinolaryngology, Bayrampasa State Hospital, Istanbul, Turkey. dr.remzidogan@gmail.com
- 3Department of Biochemistry, Bezmialem Vakif University, Istanbul, Turkey.
- 4Department of Audiology, Bezmialem Vakif University, Faculty of Health Sciences, Istanbul, Turkey.
- KMID: 2128903
- DOI: http://doi.org/10.3342/ceo.2015.8.4.312
Abstract
OBJECTIVES
In this study we investigated the probable protective effects of thymoquinone on amikacin-induced ototoxicity in rats.
METHODS
Thirty-two healthy rats were divided into four groups (amikacin, amikacin+thymoquinone, thymoquinone, and no treatment). Thymoquinone was fed to the rats via oral gavage in a dose of 40 mg/kg/day throughout the study period of 14 days. Amikacin was given by the intramuscular route in a dose of 600 mg/kg/day. Audiological assessment was conducted by the distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) tests, administered to all rats at the beginning of the study, and also on days 7 and 15. Biochemical parameters were calculated at the termination of the study to evaluate the oxidative status.
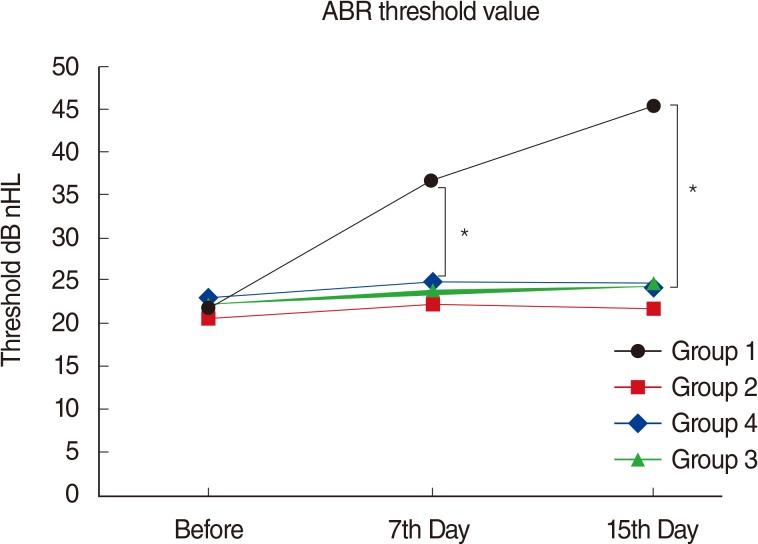
RESULTS
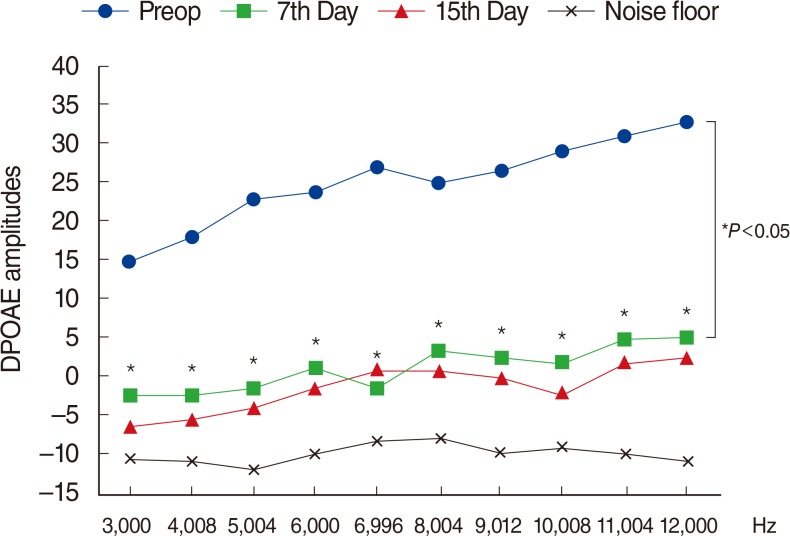
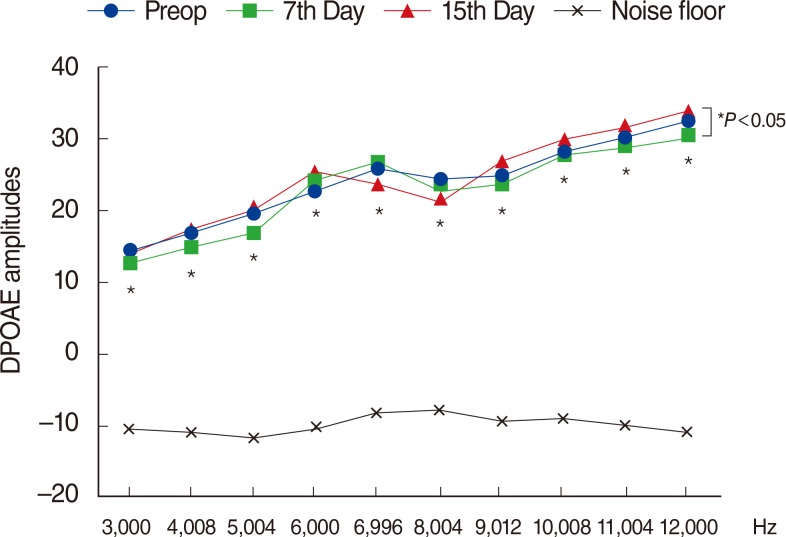
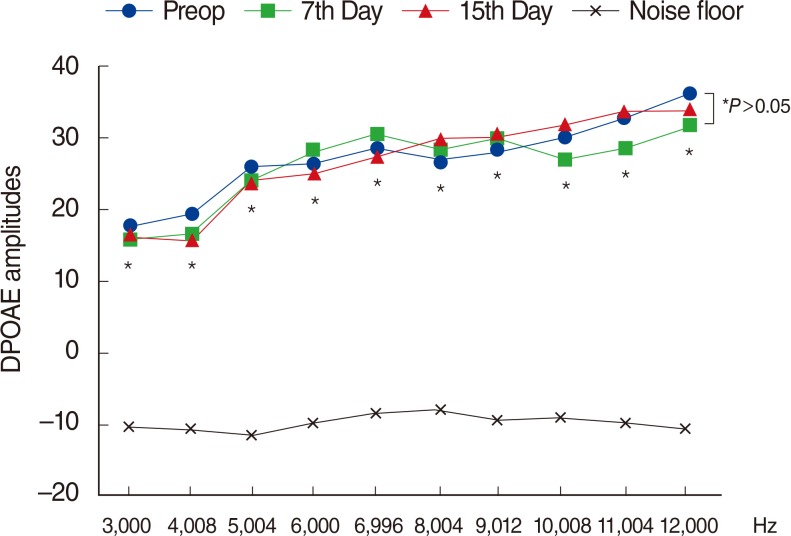
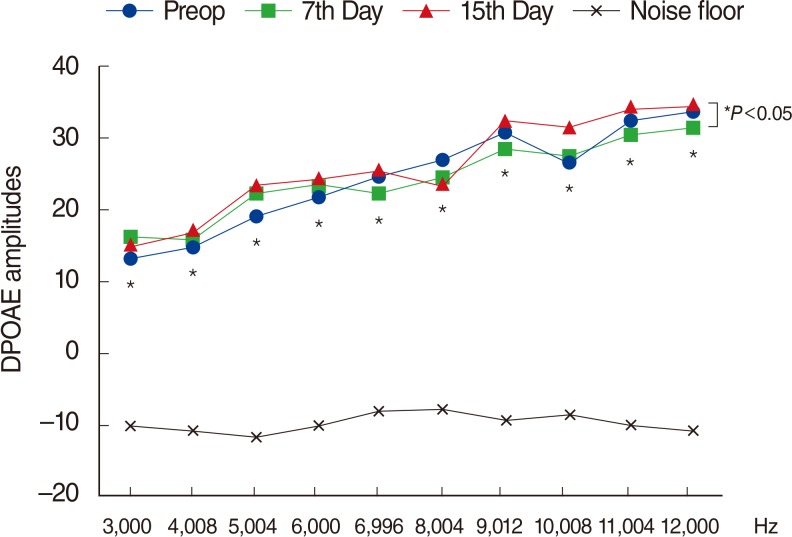
There were significant decreases in DPOAE values and significant increases in ABR thresholds of the amikacin group on days 7 and 15, as compared to the amikacin+thymoquinone group. While ABR thresholds of the amikacin group increased significantly on days 7 and 15 as compared to their initial values, there were no significant differences between the initial and the 7th and 15th day values of ABR thresholds in the amikacin+thymoquinone group. Total oxidant status and oxidative stress index values of the amikacin+thymoquinone group were significantly lower than those of the amikacin group. Total antioxidant status values of the amikacin+thymoquinone group were significantly higher than those of the amikacin group.
CONCLUSION
Our study has demonstrated that the ototoxic effect brought forth by amikacin could be overcome with the concurrent use of thymoquinone.
Keyword
Figure
Reference
-
1. Kasse CA, Hyppolito MA, Cruz OL, de Oliveira JA. Ototoxicity and otoprotection. Rev Bras Med. 2008; 4:105–115.2. Aquino TJ, Oliveira JA, Rossato M. Ototoxicity and otoprotection in the inner ear of guinea pigs using gentamicin and amikacin: ultrastructural and functional aspects. Braz J Otorhinolaryngol. 2008; Nov-Dec. 74(6):843–852. PMID: 19582340.3. Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011; 6. 41(6):441–449. PMID: 21309997.
Article4. Begg EJ, Barclay ML. Aminoglycosides: 50 years on. Br J Clin Pharmacol. 1995; 6. 39(6):597–603. PMID: 7654476.5. Murillo-Cuesta S, Contreras J, Cediel R, Varela-Nieto I. Comparison of different aminoglycoside antibiotic treatments to refine ototoxicity studies in adult mice. Lab Anim. 2010; 4. 44(2):124–131. PMID: 19858169.
Article6. Kitasato I, Yokota M, Inouye S, Igarashi M. Comparative ototoxicity of ribostamycin, dactimicin, dibekacin, kanamycin, amikacin, tobramycin, gentamicin, sisomicin and netilmicin in the inner ear of guinea pigs. Chemotherapy. 1990; 36(2):155–168. PMID: 2311443.
Article7. Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007; 10. 15(5):352–357. PMID: 17823553.
Article8. Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005; 3. 79(5):644–651. PMID: 15672440.9. Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008; 8. 22(4):447–452. PMID: 18705755.10. Ashraf SS, Rao MV, Kaneez FS, Qadri S, Al-Marzouqi AH, Chandranath IS, et al. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J Chromatogr Sci. 2011; 4. 49(4):321–326. PMID: 21439125.
Article11. Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003; 5. 26(2):87–98. PMID: 12816394.
Article12. Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000; 49(1):63–74. PMID: 10997492.13. Al-Amri AM, Bamosa AO. Phase I safety and clinical activity study of thymoquinone in patients with advanced refractory malignant disease. Shiraz E Med J. 2009; 7. 10(3):107–111.14. Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. The guide for the care and use of laboratory animals. 7th ed. Washington DC: National Academies Press;1996.15. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; 4. 37(4):277–285. PMID: 15003729.
Article16. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 12. 38(12):1103–1111. PMID: 16214125.
Article17. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan-Feb. 5(1):3–22. PMID: 10686428.
Article18. Miman MC, Ozturan O, Iraz M, Erdem T, Olmez E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear Res. 2002; 7. 169(1-2):121–129. PMID: 12121745.
Article19. Lenoir M, Puel JL. Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. II. Histological study. Hear Res. 1987; 26(2):199–209. PMID: 3570998.20. Xuan W, Dong M, Dong M. Effects of compound injection of Pyrola rotundifolia L and Astragalus membranaceus Bge on experimental guinea pigs' gentamicin ototoxicity. Ann Otol Rhinol Laryngol. 1995; 5. 104(5):374–380. PMID: 7747908.
Article21. Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C R Acad Sci III. 1993; 7. 316(7):682–687. PMID: 8019890.22. Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008; 7. 249(2-3):91–96. PMID: 18514377.
Article23. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007; 13(1):119–126. PMID: 17266591.
Article24. Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, McDonald WJ. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J Infect Dis. 1992; 6. 165(6):1026–1032. PMID: 1583319.
Article25. Stavroulaki P, Vossinakis IC, Dinopoulou D, Doudounakis S, Adamopoulos G, Apostolopoulos N. Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2002; 2. 128(2):150–155. PMID: 11843723.
Article26. Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986; 5. 77(5):1492–1500. PMID: 3700652.
Article27. Sassada MM, Ceccon ME, Navarro JM, Vaz FA. Hearing loss in newborn admitted in intensive care unit. Pediatria (Sao Paul). 2005; 27:163–171.28. Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999; 2. 128(1-2):40–44. PMID: 10082281.29. Nekrassov V, Sitges M. Vinpocetine protects from aminoglycoside antibiotic-induced hearing loss in guinea pig in vivo. Brain Res. 2000; 6. 868(2):222–229. PMID: 10854574.
Article30. Oliveira JA, Canedo DM, Rossato M, Andrade MH. Self-protection against aminoglycoside ototoxicity in guinea pigs. Otolaryngol Head Neck Surg. 2004; 9. 131(3):271–279. PMID: 15365547.
Article31. Erdem T, Ozturan O, Iraz M, Miman MC, Olmez E. Dose-dependent dual effect of melatonin on ototoxicity induced by amikacin in adult rats. Eur Arch Otorhinolaryngol. 2005; 4. 262(4):314–321. PMID: 15170574.
Article32. Lecain E, Omri B, Behar-Cohen F, Tran Ba, Crisanti P. The role of PKCzeta in amikacin-induced apoptosis in the cochlea: prevention by aspirin. Apoptosis. 2007; 2. 12(2):333–342. PMID: 17191118.33. Berkiten G, Salturk Z, Topaloglu I, Ugrad H. Protective effect of pentoxifylline on amikacin-induced ototoxicity in rats. Am J Otolaryngol. 2012; Nov-Dec. 33(6):689–692. PMID: 22784588.
Article34. Amora Lde A, Murashima Ade A, Rossato M, Moreira MB, Hyppolito MA, Fagundes DJ. The effects of hyperbaric oxygen therapy upon ototoxic injuries produced by amikacin in guinea pigs. Braz J Otorhinolaryngol. 2013; May-Jun. 79(3):342–348. PMID: 23743750.35. Bayindir T, Filiz A, Iraz M, Kaya S, Tan M, Kalcioglu MT. Evaluation of the protective effect of Beta glucan on amikacin ototoxicity using distortion product otoacoustic emission measurements in rats. Clin Exp Otorhinolaryngol. 2013; 3. 6(1):1–6. PMID: 23525870.
Article36. Pavlidis P, Maurer J, Apostolidou E, Kekes G, Kouvelas D. Memantine's action against aminoglycoside-induced ototoxicity. Eur Arch Otorhinolaryngol. 2014; 6. 271(6):1491–1496. PMID: 23917735.
Article37. Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000; 6. 97(13):7597–7602. PMID: 10861021.
Article38. Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013; 8. 270(8):2231–2237. PMID: 23161274.
Article39. Jaswal A, Sinha N, Bhadauria M, Shrivastava S, Shukla S. Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environ Toxicol Pharmacol. 2013; 11. 36(3):779–786. PMID: 23958970.
Article40. Strupp M, Arbusow V. Acute vestibulopathy. Curr Opin Neurol. 2001; 2. 14(1):11–20. PMID: 11176212.41. Klemens JJ, Meech RP, Hughes LF, Somani S, Campbell KC. Antioxidant enzyme levels inversely covary with hearing loss after amikacin treatment. J Am Acad Audiol. 2003; 4. 14(3):134–143. PMID: 12859138.
Article42. Sankaranarayanan C, Pari L. Thymoquinone ameliorates chemical induced oxidative stress and β-cell damage in experimental hyperglycemic rats. Chem Biol Interact. 2011; 4. 190(2-3):148–154. PMID: 21382363.
Article43. Nagi MN, Al-Shabanah OA, Hafez MM, Sayed-Ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011; May-Jun. 25(3):135–142. PMID: 20957680.
Article44. World Health Organization, Programme for the Prevention of Deafness and Hearing Impairment (PDH). Report of an informal consultation on strategies for prevention of hearing impairment from ototoxic drugs. Geneva: World Health Organization;1994.45. Infectious Diseases Society of America. The 10 × '20 Initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010; 4. 50(8):1081–1083. PMID: 20214473.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Protective Effect of Beta Glucan on Amikacin Ototoxicity Using Distortion Product Otoacoustic Emission Measurements in Rats
- Is Hyperbaric Oxygen Therapy Effective in Cisplatin-Induced Ototoxicity in Rats?
- Effectiveness of thymoquinone, zeolite, and platelet-rich plasma in model of corrosive oesophagitis induced in rats
- Immunohistochemical Expressions of TrkB and TrkC Receptors in Rat Cochleas with Amikacin-induced Ototoxicity
- Antinociceptive Effects of Amikacin on Neuropathic Pain in Rats