J Adv Prosthodont.
2015 Apr;7(2):98-107. 10.4047/jap.2015.7.2.98.
Investigation of flexural strength and cytotoxicity of acrylic resin copolymers by using different polymerization methods
- Affiliations
-
- 1Department of Prosthodontics, Faculty of Dentistry, Bulent Ecevit University, Zonguldak, Turkey. sonurs60@hotmail.com
- 2Department of Prosthodontics, Faculty of Dentistry, Cumhuriyet University, Sivas, Turkey.
- 3Department of Prosthodontics, Dentaforum Dental Clinic, Kayseri, Turkey.
- 4Department of Chemistry, Tunceli University, Tunceli, Turkey.
- 5Department of Microbiology Faculty of Medical, Cumhuriyet University, Sivas, Turkey.
- KMID: 2118239
- DOI: http://doi.org/10.4047/jap.2015.7.2.98
Abstract
- PURPOSE
The aim of this study was to appraise the some mechanical properties of polymethyl methacrylate based denture base resin polymerized by copolymerization mechanism, and to investigate the cytotoxic effect of these copolymer resins.
MATERIALS AND METHODS
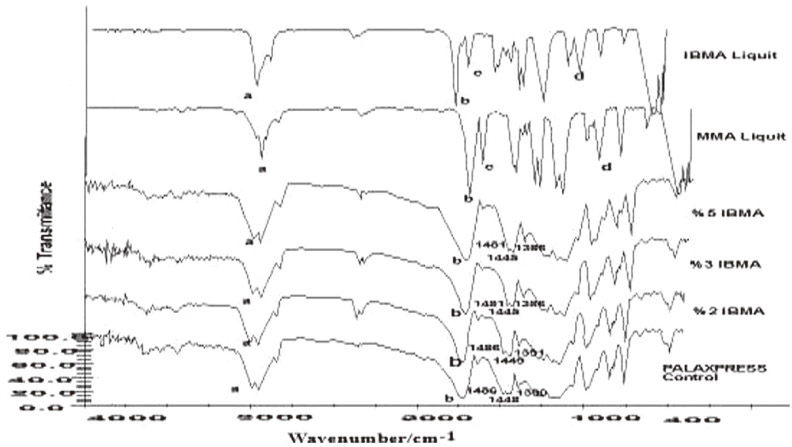
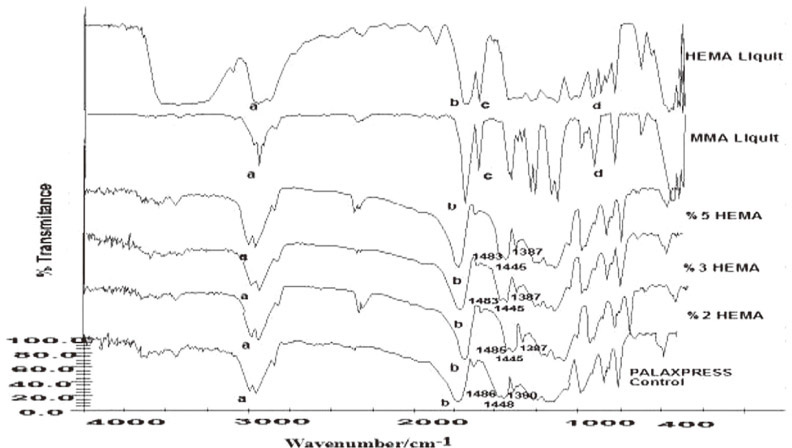
2-hydroxyethyl methacrylate (HEMA) and isobutyl methacrylate (IBMA) were added to monomers of conventional heat polymerized and injection-molded poly methyl methacrylate (PMMA) resin contents of 2%, 3%, and 5% by volume and polymerization was carried out. Three-point bending test was performed to detect flexural strength and the elasticity modulus of the resins. To determine the statistical differences between the study groups, the Kruskall-Wallis test was performed. Then pairwise comparisons were performed between significant groups by Mann-Whitney U test. Agar-overlay test was performed to determine cytotoxic effect of copolymer resins. Chemical analysis was determined by FTIR spectrum.
RESULTS
Synthesis of the copolymer was approved by FTIR spectroscopy. Within the conventional heat-polymerized group maximum transverse strength had been seen in the HEMA 2% concentration; however, when the concentration ratio increased, the strength decreased. In the injection-molded group, maximum transverse strength had been seen in the IBMA 2% concentration; also as the concentration ratio increased, the strength decreased. Only IBMA showed no cytotoxic effect at low concentrations when both two polymerization methods applied while HEMA showed cytotoxic effect in the injection-molded resins.
CONCLUSION
Within the limitations of this study, it may be concluded that IBMA and HEMA may be used in low concentration and at high temperature to obtain non-cytotoxic and durable copolymer structure.
Keyword
MeSH Terms
Figure
Reference
-
1. Jagger DC, Harrison A, Jandt KD. The reinforcement of dentures. J Oral Rehabil. 1999; 26:185–194.2. Tamareselvy K, Rueggeberg FA. Dynamic mechanical analysis of two crosslinked copolymer systems. Dent Mater. 1994; 10:290–297.3. Rached RN, Del-Bel Cury AA. Heat-cured acrylic resin repaired with microwave-cured one: bond strength and surface texture. J Oral Rehabil. 2001; 28:370–375.4. Cunha TR, Regis RR, Bonatti MR, de Souza RF. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009; 17:103–107.5. Arlen MJ, Dadmun MD. The reinforcement of polystyrene and poly(methyl methacrylate) interfaces using alternating copolymers. Polymer. 2003; 44:6883–6889.6. Sperling LH. Polymeric multicomponent materials: an introduction. New York; NY: Wiley;1997.7. Moszner N, Fischer UK, Angermann J, Rheinberger V. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater. 2006; 22:1157–1162.8. Ko MJ, Kim SH, Jo WH. The effects of copolymer architecture on phase separation dynamics of immiscible homopolymer blends in the presence of copolymer: a Monte Carlo simulation. A Monte Carlo simulation, Polymer. 2000; 4:6387–6394.9. Doğan OM, Bolayır G, Keskin S, Boztuğ A, Doğan A, Bek B. Effects of some methacrylate monomers used as liquid component on tensile and flexural strengths of poly (methyl-methacrylate) denture base resin. Mater Res Innov. 2006; 10:424–427.10. Umemoto K, Kurata S, Morishita K, Kawase T. Basic study of a new soft resin applied with bisfunctional siloxane oligomer. Dent Mater J. 2007; 26:656–658.11. Keyf F, Uzun G, Mutlu M. The effects of HEMA-monomer and air atmosphere treatment of glass fibre on the transverse strength of a provisional fixed partial denture resin. J Oral Rehabil. 2003; 30:1142–1148.12. Rodford RA. Further development and evaluation of high impact strength denture base materials. J Dent. 1990; 18:151–157.13. Brar AS, Hooda S, Goyal AK. Microstructure determination of 2-hydroxy ethyl methacrylate and methyl acrylate copolymers by NMR spectroscopy. J Mol Struc. 2006; 828:25–37.14. Isaksson M, Lindberg M, Sundberg K, Hallander A, Bruze M. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005; 53:292–297.15. Yoshii E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res. 1997; 37:517–524.16. Park JB. Biomaterials. In : Bronzino JD, editor. Biomedical Engineering Handbook. Boca Raton: CRC Press and IEEE Press;1995. p. 530–610.17. Kedjarune U, Charoenworaluk N, Koontongkaew S. Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J. 1999; 44:25–30.18. Lefebvre CA, Knoernschild KL, Schuster GS. Cytotoxicity of eluates from light-polymerized denture base resins. J Prosthet Dent. 1994; 72:644–650.19. Harrison A, Huggett R. Effect of the curing cycle on residual monomer levels of acrylic resin denture base polymers. J Dent. 1992; 20:370–374.20. Yunus N, Harrison A, Huggett R. Effect of microwave irradiation on the flexural strength and residual monomer levels of an acrylic resin repair material. J Oral Rehabil. 1994; 21:641–648.21. American dental Association (ADA). Specifications for denture base polymer number 12. 1999. Reaffirmed 2008.22. ISO 1567:1999. Dentistry - Denture base polymers. Switzerland: International Standard Organization. ISO Geneva;1999.23. Schmalz G. Agar overlay method. Int Endod J. 1988; 21:59–66.24. Jagger D, Harrison A, Jagger R, Milward P. The effect of the addition of poly(methyl methacrylate) fibres on some properties of high strength heat-cured acrylic resin denture base material. J Oral Rehabil. 2003; 30:231–235.25. Rodford RA, Braden M. Further observations on high impact strength denture-base materials. Biomaterials. 1992; 13:726–728.26. Cho K, Ahn TO, Ryu HS, Seo KH. Mechanical effects according to the type of poly(styrene-co-methyl methacrylate) copolymers at polystyrene/poly(methyl methacrylate) interfaces. Polymer. 1996; 37:4849–4852.27. Hayden WJ. Flexural strength of microwave-cured denture baseplates. Gen Dent. 1986; 34:367–371.28. Craig RG. Restorative dental materials. 9th ed. St. Louis: Mosby;1989.29. Cho K, Yang JH, Park CE. The effect of interfacial adhesion on toughening behaviour of rubber modified poly(methyl methacrylate). Polymer. 1997; 38:5161–5167.30. Johnson JA, Jones DW. The mechanical properties of PMMA and its copolymers with ethyl methacrylate and butyl methacrylate. J Mater Sci. 1994; 29:870–876.31. Clarke RL. Dynamic mechanical thermal analysis of dental polymers. III. Heterocyclic methacrylates. Biomaterials. 1989; 10:630–633.32. Doğan OM, Bolayır G, Boztuğ A, Turgut M, Zengin HB. The effects of maleic anhydride terpolymer and its ester derivatives on tensile bond strength between the acrylic resin and resilient lining material. J App Polym Sci. 2007; 104:1338–1341.33. Cerveny S, Goyanes SN, Marzocca AJ, Rubiolo GH. Dynamic properties in aluminum filled PMMA. Polymer. 1999; 40:1495–1500.34. Jorge JH, Giampaolo ET, Machado AL, Vergani CE. Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent. 2003; 90:190–193.35. Kalipçilar B, Karaağaçlioğlu L, Hasanreisoğlu U. Evaluation of the level of residual monomer in acrylic denture base materials having different polymerization properties. J Oral Rehabil. 1991; 18:399–401.36. Schmalz G. Use of cell cultures for toxicity testing of dental materials-advantages and limitations. J Dent. 1994; 22:S6–S11.37. Sheridan PJ, Koka S, Ewoldsen NO, Lefebvre CA, Lavin MT. Cytotoxicity of denture base resins. Int J Prosthodont. 1997; 10:73–77.38. Cimpan MR, Cressey LI, Skaug N, Halstensen A, Lie SA, Gjertsen BT, Matre R. Patterns of cell death induced by eluates from denture base acrylic resins in U-937 human monoblastoid cells. Eur J Oral Sci. 2000; 108:59–69.39. Polat TN, Karacaer O, Tezvergil A, Lassila LV, Vallittu PK. Water sorption, solubility and dimensional changes of denture base polymers reinforced with short glass fibers. J Biomater Appl. 2003; 17:321–335.40. Phoenix RD, Mansueto MA, Ackerman NA, Jones RE. Evaluation of mechanical and thermal properties of commonly used denture base resins. J Prosthodont. 2004; 13:17–27.41. Sipahi C, Özen J, Ural AU, Dalkız M, Beydemir B. Effects of five different acrylic resin denture base materials on gingival fibroblasts. Gulhane Med J. 2005; 47:275–278.42. Ergun G, Sagesen LM, Dogan A, Ozkul A, Demirel E. Examination of cytotoxicity of denture base resins by agar diffusion and fitler diffusion test methods. Acta Odontol Turc. 2006; 23:31–37.43. Urban VM, Cass QB, Oliveira RV, Giampaolo ET, Machado AL. Development and application of methods for determination of residual monomer in dental acrylic resins using high performance liquid chromatography. Biomed Chromatogr. 2006; 20:369–376.44. Taira M, Nakao H, Matsumoto T, Takahashi J. Cytotoxic effect of methyl methacrylate on 4 cultured fibroblasts. Int J Prosthodont. 2000; 13:311–315.45. Doğan A, Bek B, Cevik NN, Usanmaz A. The effect of preparation conditions of acrylic denture base materials on the level of residual monomer, mechanical properties and water absorption. J Dent. 1995; 23:313–318.46. Jorge JH, Giampaolo ET, Machado AL, Vergani CE. Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent. 2003; 90:190–193.47. Phillips RW. Skinner's science of dental materials. 8th ed. Philadelphia: WB. Saunders Co.;1982.48. Bartoloni JA, Murchison DF, Wofford DT, Sarkar NK. Degree of conversion in denture base materials for varied polymerization techniques. J Oral Rehabil. 2000; 27:488–493.49. Bayraktar G, Guvener B, Bural C, Uresin Y. Influence of polymerization method, curing process, and length of time of storage in water on the residual methyl methacrylate content in dental acrylic resins. J Biomed Mater Res B Appl Biomater. 2006; 76:340–345.50. Truong VT, Thomasz FG. Comparison of denture acrylic resins cured by boiling water and microwave energy. Aust Dent J. 1988; 33:201–204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of polymethyl methacrylate resin mechanical properties with incorporated halloysite nanotubes
- Comparative study of flexural strength of temporary restorative resin according to surface polishing and fabrication methods
- Evaluation of Mechanical Properties of Threedimensional Printed Flexible Denture Resin according to Post-polymerization Conditions: A Pilot Study
- Effect of film thickness of resin cement on bonding efficiency in indirect composite restoration
- Effects of the color components of light-cured composite resin before and after polymerization on degree of conversion and flexural strength