Lab Anim Res.
2010 Dec;26(4):353-359. 10.5625/lar.2010.26.4.353.
Antifungal Effects of New Synthetic Materials, KAF-200522 and KAF-200522-HCl, on in vitro and in vivo Models
- Affiliations
-
- 1Institute for the 3Rs & Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul, Korea. labvet@konkuk.ac.kr
- 2Preclinical Research Center, Chemon Inc., Yongin, Korea.
- KMID: 2114703
- DOI: http://doi.org/10.5625/lar.2010.26.4.353
Abstract
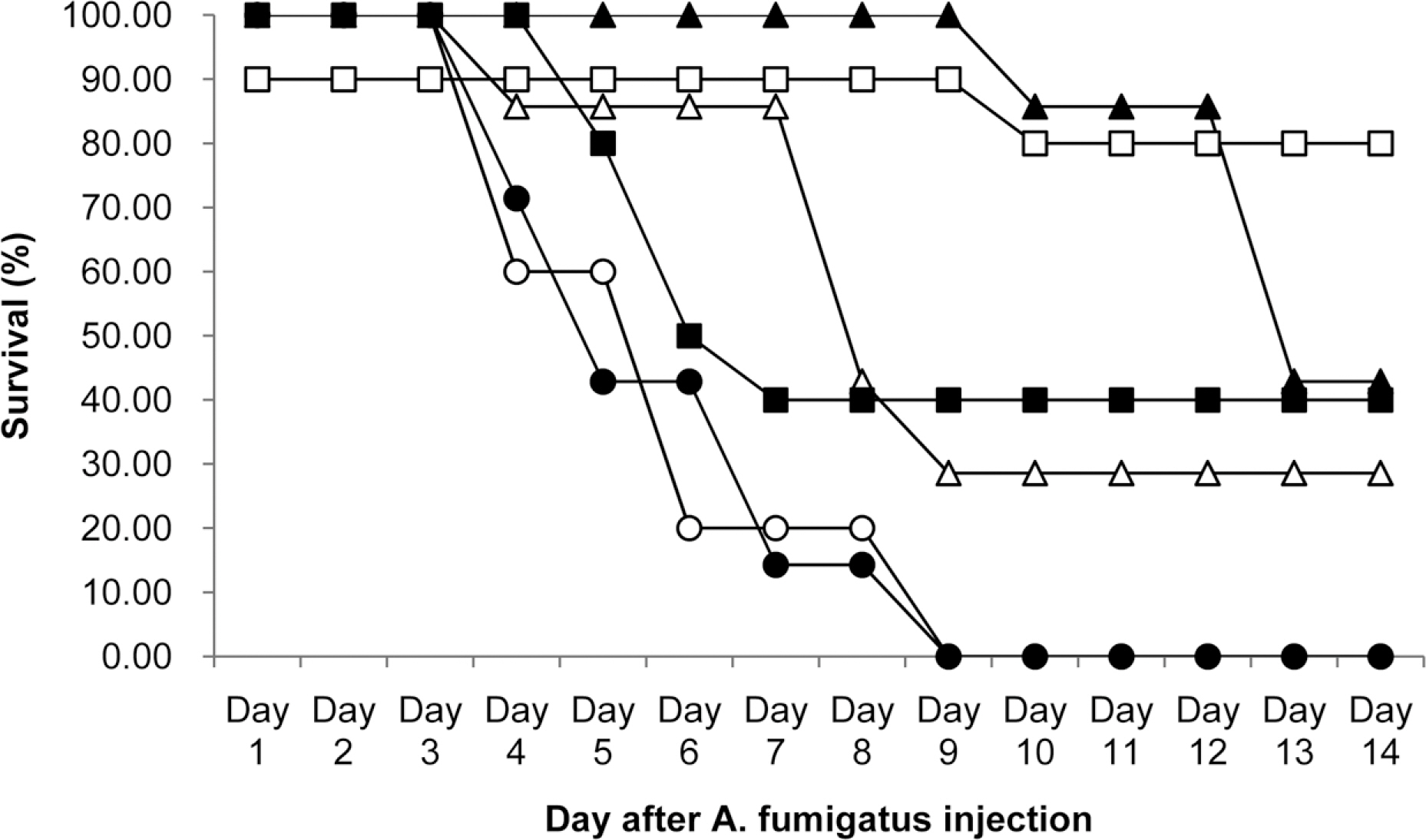
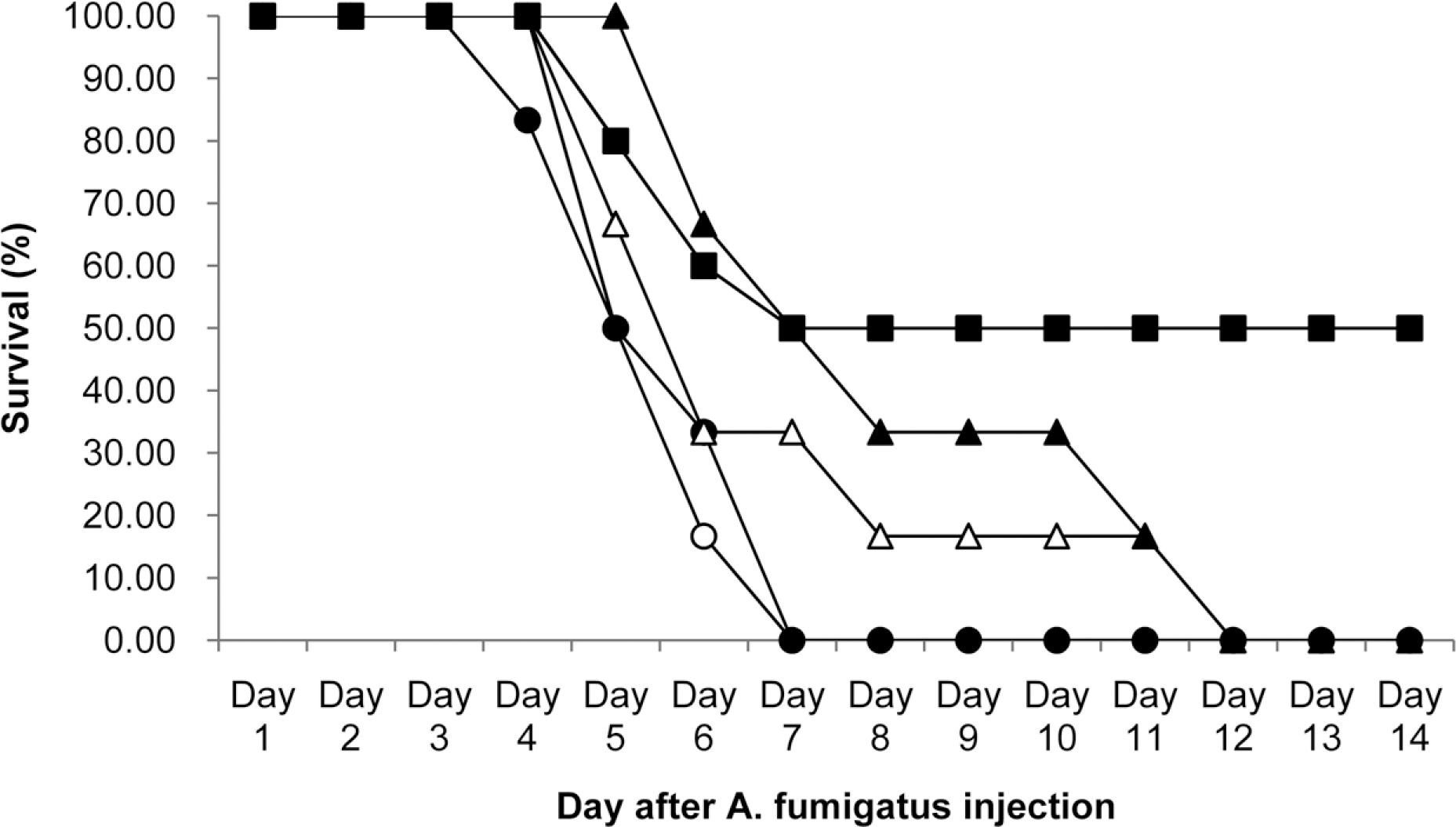
- KAF-200522 and its chloride form, KAF-200522-HCl, were invented in Chemon inc. as new triazole antifungal agents with excellent activities in vivo and in vitro against wide range of fungi. As a result of in vitro susceptibility measurements, 80% minimum inhibitory concentrations (MIC80) of both test articles against Candida albican sp. and Aspergillus fumigatus sp. were below 0.0156 microg/mL, which were over 4,100 times lower than those of fluconazole against fluconazole resistant C. albican sp. and A. fumigatus sp., and were over 16 times lower than those of amphotericin B against above same fungi. Additionally, against representative dermatophytes, Trichophyton sp., the MIC80s of both test articles were below 0.0156 microg/mL which were over 64 times lower than those of fluconazole and amphotericin B. As in vivo antifungal activities in A. fumigatus sp. infected mouse models, KAF-200522 treatment group at 600 mg/kg showed 80% survival rate which was 2 times higher than that of amphotericin B and showed 13.7 days in the mean survival time (MST) which was about 2.1 times higher than that of amphotericin B. But in KAF-200522-HCl treatment groups, all animals were found dead in contrast to 40% survival rate in amphotericin B treatment group, however dose dependent increases in MST was revealed. In conclusion, antifungal activities of KAF-200522 and its mimics, KAF-200522-HCl in vitro and in vivo were confirmed in this study, therefore the potentiality of the present compounds to be developed into new antifungal drug was expected.
Keyword
MeSH Terms
Figure
Reference
-
References
Andriole, V.T. (. 2000. ). Current and future antifungal therapy: new targets for antifungal agents. J Antimicrob Chemother. 44:151–162.Arathoon, E.G. (. 2001. ). Clinical efficacy of echinocandin antifungals. Curr Opin Infect Dis. 14:685–691.Butler, M.S. (. 2004. ). The role of natural product chemistry on drug discovery. J. Nat. Prod. 67:2131–2153.Butler, M.S. (. 2005. ). Natural products to drugs: natural product derived compounds on clinical trials. Nat. Prod. Rep. 22:162–195.Calvet, H.M., Yeaman, M.R., Filler, S.G. (. 1997. ). Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob. Agents Chemother. 41:535–539.Chomnawang, W.T., Surassmo, S., Nukoolkarn, V.S., Gritasnapan, W. (. 2005. ). Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 101:330–333.Cornely, O.A., Ullmann, A.J., Carthaus, M.(2003) Evidencebased assessment of primary antifungal prophylaxis in patients with hematologic malignancies. Blood. 101:3365–3372.Fromtling, R.A. (. 1997. ). Recent trends in the discovery, development and evaluation of antifungak agentsm. J. R. Prous Science Publisher. pp 223. Georgopapadakou, N.H., Walsh, T.J. (1994) Human mycoses: drugs and targets for emerging pathogens. Science. 264:371–373.Grant, S.M., Clissold, S.P. (. 1990. ). Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 39(6):877–916.Gupta, A.K., Sauder, D.N., Shear, N.H. (. 1992. ). Antifugal agents: An overview. Part II. J am Acad Dermarol. 206:2–7.Konishi, M., Nishio, M., Saitoh, K., Miyaki, T., Oki, T., Kawaguchi, H. (. 1989. ). Cispentacin, a new antifungal antibiotic. I. Production, isolation, physicochemical properties and structure. J. Antibiotics. 42:1749–1755.Pfaller, M.A., Bartlett, M.S., Espinel-Ingroff, A. (. 1997. ). Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi Proposed standard. NCCLS. 18(13):1–21.Ruhnke, M., Schmidt-Westhausen, A., Engelmann, E., Trautmann. (. 1996. ). Comparative evaluation of three antifungal susceptibility test methods for Candida albicans isolates and correlation with response to fluconazole therapy. J. Clin. Microbiol.3208–3211.Shadomy, S., Shadomy, H.J., Wagmer, G.E. (. 1997. ). Antifungal compounds, In Siegel, M. R. and HughI, D. S. (eds.), Dekker. pp 437. Tawara, S., Matsumoto, S., Hirose, T., Matsumoto, Y., Nakamoto, S., Mitsuno, M. (1989) In vitro antifungal synergism between pyrrolnitrin and clotrimazole. Med. Mycol.202–210.Tortorano, A.M., Viviani, M.A., Barchiesi, F., Arzeni, D., Rigoni, A.L., Cogliat, M. (. 1989. ). Comparison of three methods for testing azole susceptibilities of Candida albicans strains isolated sequentially from oral cavities of AIDS patients. J. Clin. Microbiol.1578–1583.Walsh, T.J., Hiemenz, J.W., Seibel, N.L. (. 1989. ). Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 26:1383–1396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetic and Toxicokinetic Studies of Potential Antifungal Compounds, KAF-200522 and KAF-200522 . HCl, in Animal Models
- INHIBITORY EFFECT OF ANTIFUNGAL AGENTS INCORPORATED IN DENTURE LINING MATERIALS AGAINST CANDIDA ALBICANS
- A Survey of Health-related Lifestyle of the ROKAF Pilots
- A Comparative Study of Tensile Strength in Vitro and Histologic Reaction on Rabbits between TRISORB Suture Materials and Traditional Suture Materials ( Dexon , Vicryl , Medifit )
- In Vitro Susceptibility Test of Trichophyton rubrum Against Oral Antifungal Agents