Lab Anim Res.
2010 Dec;26(4):331-337. 10.5625/lar.2010.26.4.331.
Functional Analysis of TRPV6 Polymorphisms
- Affiliations
-
- 1Division of Longevity and Biofunctional Medicine, Pusan National University School of Korean Medicine, Yangsan, Korea. vision@pusan.ac.kr
- 2Department of Physiology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2114700
- DOI: http://doi.org/10.5625/lar.2010.26.4.331
Abstract
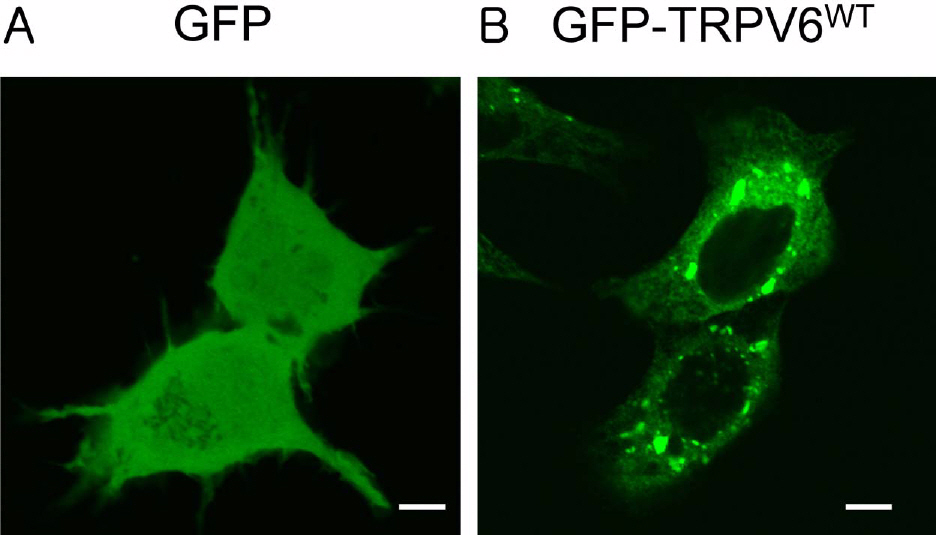
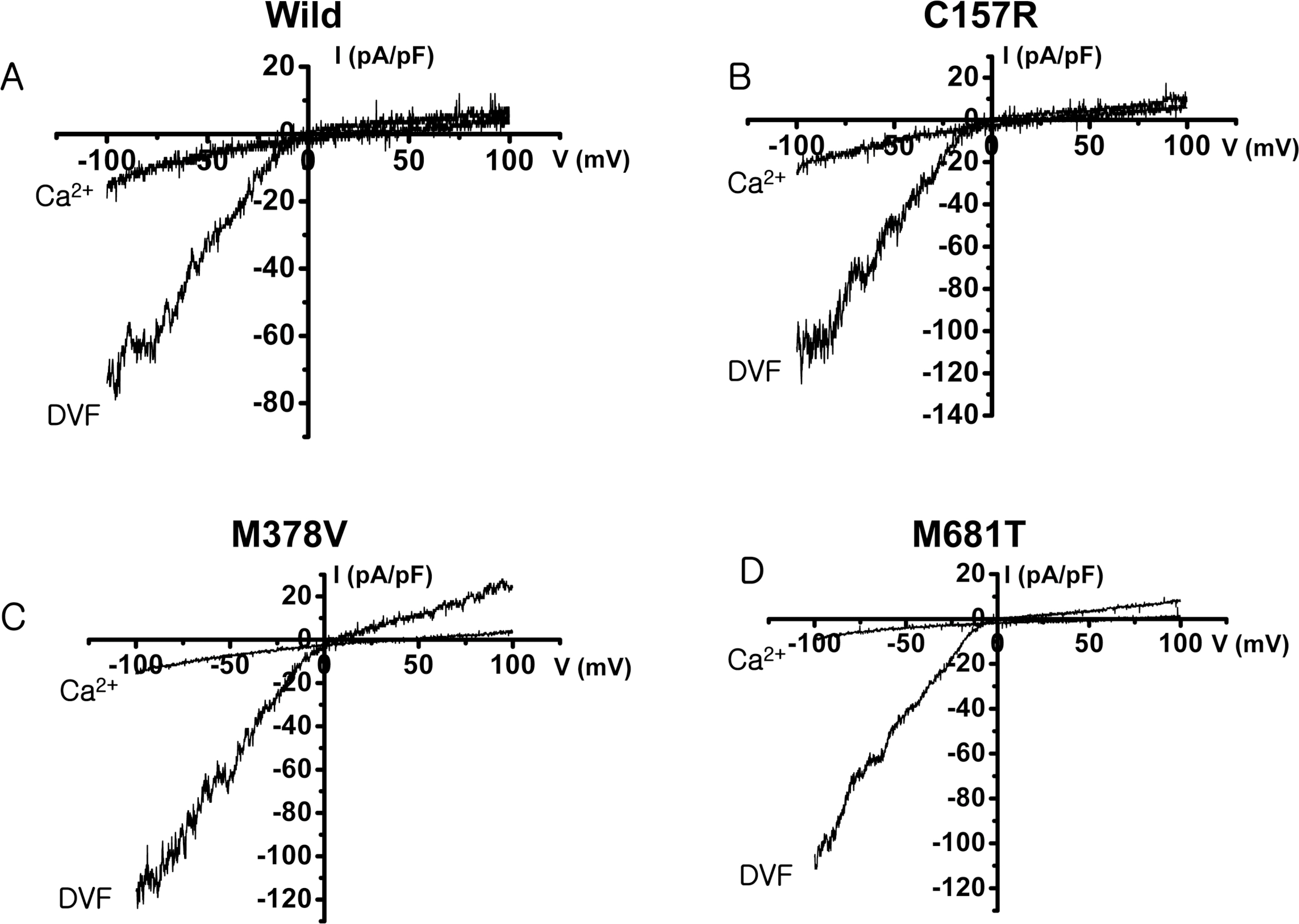
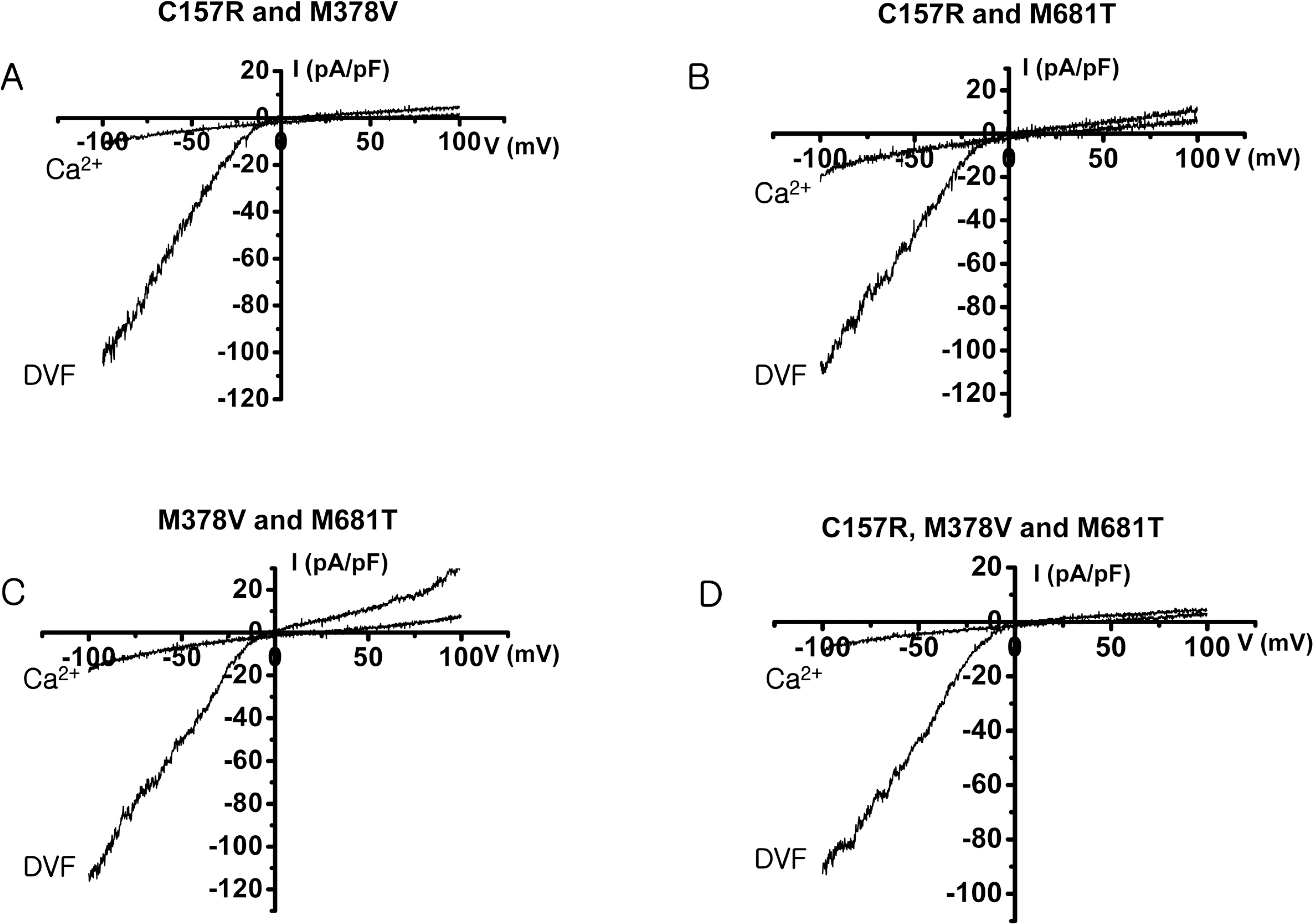
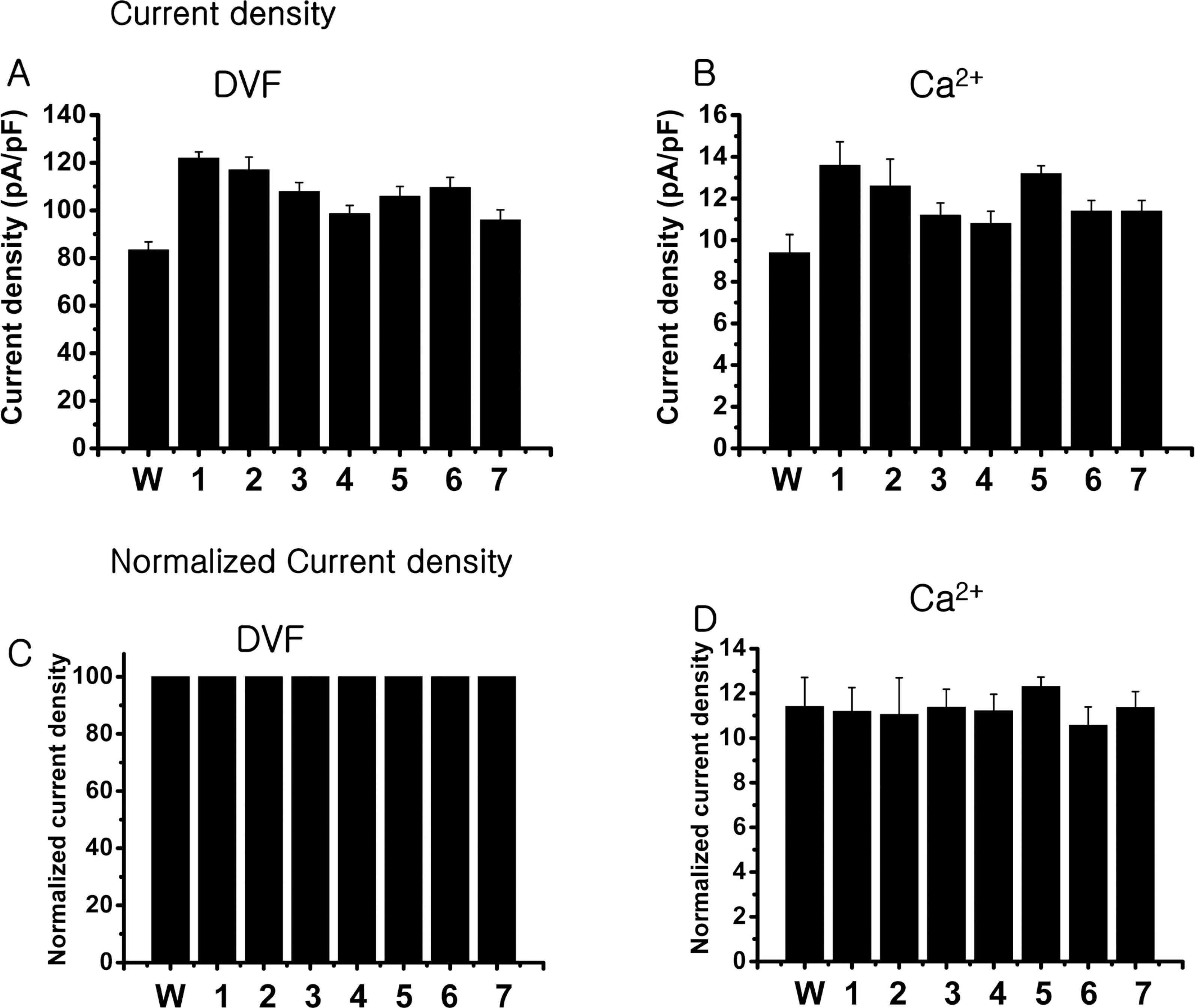
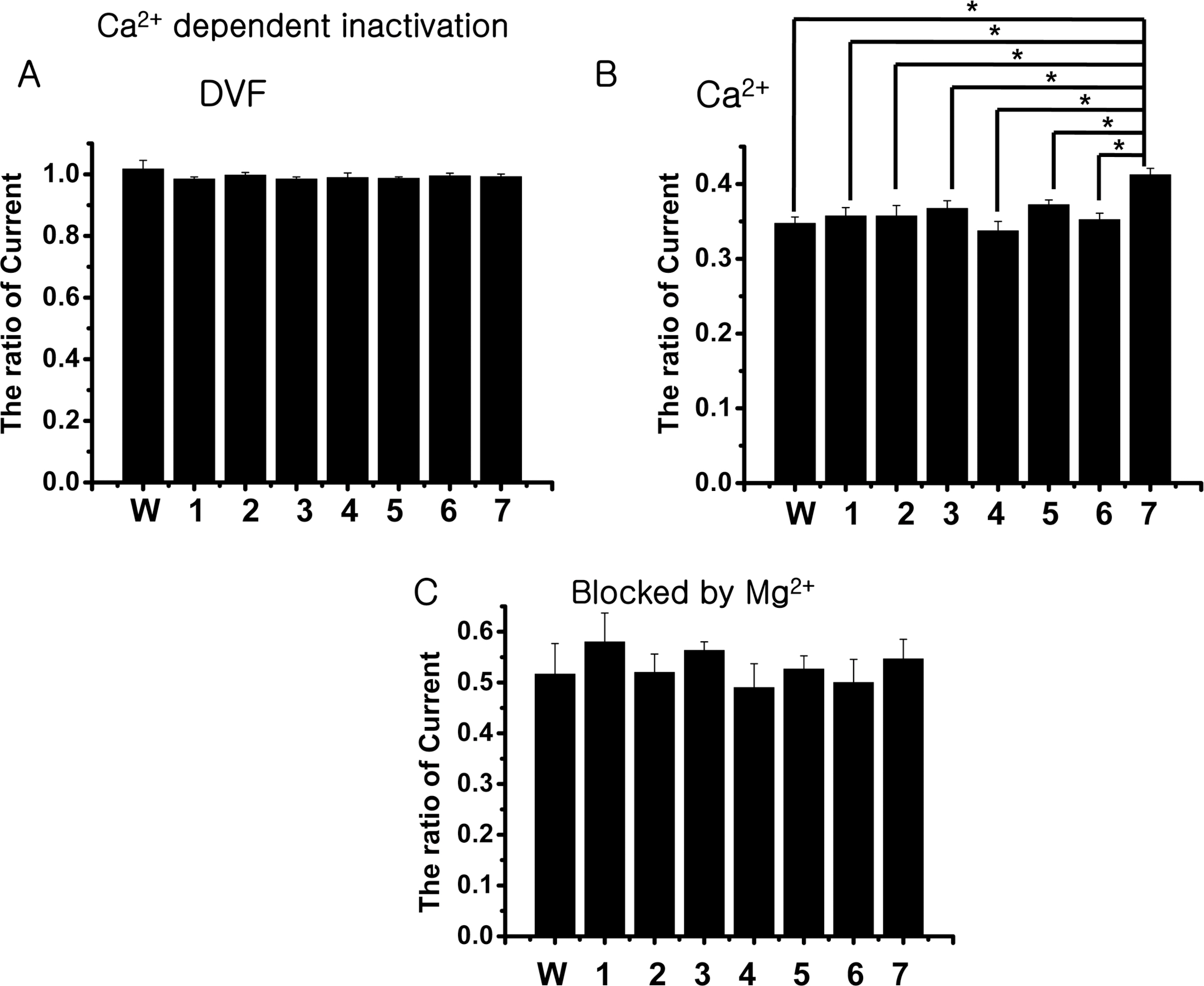
- The rate-limiting step of dietary calcium absorption in the intestine requires the brush border calcium entry channel transient receptor potential vanilloid 6 (TRPV6). The putatively-selected TRPV6 haplotype contains three candidate sites for functional differences, namely derived non-synonymous substitutions C157R, M378V and M681T. Functional electrophysiological characteristics between wild-type and mutant (C157R, M378V and M681T) TRPV6 proteins were investigated by cloning the mutant TRPV6 forms, transfecting cell lines, and carrying out electrophysiology experiments via patch clamp analysis. No statistically significant differences in biophysical channel function were found although one property, namely Ca2+-dependent inactivation, may show functionally-relevant differences between the wild-type and mutant TRPV6 proteins. This study shows that Ca2+-dependent inactivation is one of the good differentiation characteristics in TRPV6, and will be useful in an advancing our knowledge about TRPV6.
MeSH Terms
Figure
Reference
-
References
Altshuler, D., Brooks, L.D., Chakravarti, A., Collins, F.S., Daly, M.J. and Donnelly, P. (. 2005. ). International HapMap-Consortium A haplotype map of the human genome. Nature. 437(7063):1299–1320.Akey, J.M., Eberle, M.A., Rieder, M.J., Carlson, C.S., Shriver, M.D., Nickerson, D.A. and Kruglyak, L. (. 2004. ). Population history and natural selection shape patterns of genetic variation in 132 Genes. PLoS Biol. 2, e286. Akey, J.M., Swanson, W.J., Madeoy, J., Eberle, M. and Shriver, M.D. (2006) TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum. Mol. Genet. 15(13):2106–2113.Birnbaumer, L., Yildirim, E., Abramowitz, J. and Yidirim, E. (. 2003. ). A comparison of the genes coding for canonical TRP channels and their M, V and P relatives. Cell Calcium. 33(5–6):419–432.Clapham, D.E. (. 2003. ). TRP channels as cellular sensors. Nature. 426(6966):517–524.Erler, I., Hirnet, D., Wissenbach, U., Flockerzi, V. and Niemeyer, B.A. (. 2004. ). Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J. Biol. Chem. 279(33):34456–34463.Hinds, D.A., Stuve, L.L., Nilsen, G.B., Halperin, E., Eskin, E., Ballinger, D.G., Frazer, K.A. and Cox, D.R. (. 2005. ). Whole-genome patterns of common DNA variation in three human populations. Science. 307(5712):1072–1079.Hoenderop, J.G., Nilius, B. and Bindels, R.J. (. 2005. ). Calcium absorption across epithelia. Physiol. Rev. 85(1):373–422.Hoenderop, J.G., Vennekens, R., Muller, D., Prenen, J. and Droogmans, G. (. 2001. ). Function and expression of the epithelial Ca2+ channel family: comparison of the epithelial Ca2+ channel 1 and 2. J. Physiol. (Lond.). 537(3):747–761.Hughes, D.A., Tang, K., Strotmann, R., Schöneberg, T., Prenen, J., Nilius, B. and Stoneking, M. (. 2008. ). Parallel selection on TRPV6 in human populations. PLoS ONE. 3(2):e1686.Montell, C., Birnbaumer, L. and Flockerzi, V. (. 2002. ). The TRP channels, a remarkably functional family. Cell. 108(5):595–598.Niemeyer, B.A., Bergs, C., Wissenbach, U., Flockerzi, V. and Trost, C. (. 2001. ). Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc. Natl. Acad. Sci. USA. 98(6):3600–3605.Nijenhuis, T., Hoenderop, J.G., Nilius, B. and Bindels, R.J. (. 2003. ). Pathophysiological implications of the novel epithelial Ca2+ channels TRPV5 and TRPV6. Pflugers Arch. 446(4):401–409.Nilius, B., Prenen, J., Hoenderop, J.G., Vennekens, R., Hoefs, S., Weidema, A.F., Droogmans, G. and Bindels, R.J. (. 2002. ). Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J. Biol. Chem. 277(34):30852–30858.Nilius, B., Prenen, J., Vennekens, R., Hoenderop, J.G., Bindels, R.J., Droogmans, G. and Nilius, B. (. 2001a. ). Modulation of the epithelial calcium channel, ECaC, by intracellular Ca2+. Cell Calcium. 29(6):417–428.Nilius, B., Vennekens, R., Prenen, J., Hoenderop, J.G., Bindels, R.J. and Droogmans, G. (. 2000. ). Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J. Physiol. Lond. 527(2):239–248.Nilius, B., Vennekens, R., Prenen, J., Hoenderop, J.G., Droogmans, G. and Bindels, R.J. (. 2001b. ). The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J. Biol. Chem. 276(2):1020–1025.Nilius, B., Weidema, F., Prenen, J., Hoenderop, J.G., Vennekens, R., Hoefs, S., Droogmans, G. and Bindels, R.J. (. 2003. ). The carboxyl terminus of the epithelial Ca2+ channel ECaC1 is involved in Ca2+-dependent inactivation. Pflugers Arch. 445(5):584–588.Stajich, J.E. and Hahn, M.W. (. 2005. ). Disentangling the effects of demography and selection in human history. Mol. Biol. Evol. 22(1):63–73.Suzuki, Y., Pasch, A., Bonny, O., Mohaupt, M.G., Hediger, M.A. and Frey, F.J. (. 2008. ). Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum. Mol. Genet. 17(11):1613–1618.van de Graaf. S.F., Boullart, I., Hoenderop, J.G. and Bindels, R.J. (. 2004. ). Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1-alpha, 25-dihydroxy vitamin D3 and dietary Ca2+. J. Steroid Biochem. Mol. Biol. 89–90(1–5):303–308.van de Graaf, S.F., Hoenderop, J.G., Gkika, D., Lamers, D., Prenen, J., Rescher, U., Gerke, V., Staub, O., Nilius, B. and Bindels, R.J. (. 2003. ). Functional expression of the epithelial Ca2+ channels TRPV5 and TRPV6 requires association of the S100A10–annexin 2 complex. EMBO J. 22(7):1478–1487.Vennekens, R., Hoenderop, J.G., Prenen, J., Stuiver, M., Willems, P.H., Droogmans, G., Nilius, B. and Bindels, R.J. (. 2000. ). Permeation and gating properties of the novel epithelial Ca2+ channel. J. Biol. Chem. 275(6):3963–3969.Vennekens, R., Prenen, J., Hoenderop, J.G., Bindels, R.J., Droogmans, G. and Nilius, B. (. 2001. ). Pore properties and ionic block of the rabbit epithelial calcium channel expressed in HEK 293 cells. J. Physiol. 530(2):183–191.Vezzoli, G., Terranegra, A., Arcidiacono, T., Biasion, R., Coviello, D., Syren, M.L., Paloschi, V., Giannini, S., Mignogna, G., Rubinacci, A., Ferraretto, A., Cusi, D., Bianchi, G. and Soldati, L. (. 2007. ). R990G polymorphism of calcium-sensing receptor does produce a gain-of-function and predispose to primary hypercalciuria. Kidney Int. 71(11):1155–1162.Voets, T., Janssens, A., Droogmans, G. and Nilius, B. (. 2003a. ). A single pore residue determines calcium selectivity, inward rectification and pore size of TRPV6 (CaT1/ECaC2). Biophys. J. (Annual Meeting Abstracts) 2724-Pos. Voets, T., Janssens, A., Prenen, J., Droogmans, D., Nilius, G. (2003b) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J. Gen. Physiol. 121(3):245–260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RNA interference mediated suppression of TRPV6 inhibits the progression of prostate cancer in vitro by modulating cathepsin B and MMP9 expression
- No Association Between Functional Polymorphisms in COMT and MTHFR and Schizophrenia Risk in Korean Population
- Association between Polymorphisms in Toll-like Receptor 9 Gene and Outcomes after Ischemic Stroke
- Polymorphisms of the Serotonin Transporter Gene and G-Protein beta3 Subunit Gene in Korean Children with Irritable Bowel Syndrome and Functional Dyspepsia
- Interaction between thyroglobulin and ADAMTS16 in premature ovarian failure