Tuberc Respir Dis.
2007 Feb;62(2):134-139. 10.4046/trd.2007.62.2.134.
Gefitinib-Related Interstitial Pneumonia
- Affiliations
-
- 1Department of Internal Medicine, Korea Cancer Center Hospital, Korea. jclee@kcch.re.kr
- 2Department of Radiology, Korea Cancer Center Hospital, Korea.
- 3Department of Internal Medicine, Kangnam St. Mary's Hospital, Seoul, Korea.
- KMID: 2114601
- DOI: http://doi.org/10.4046/trd.2007.62.2.134
Abstract
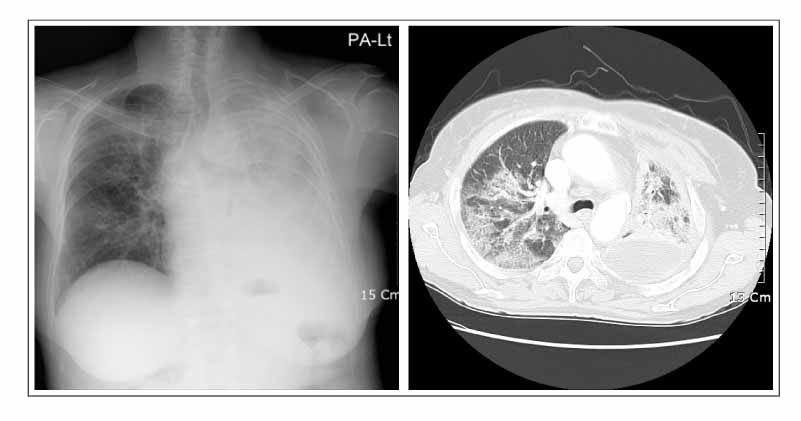
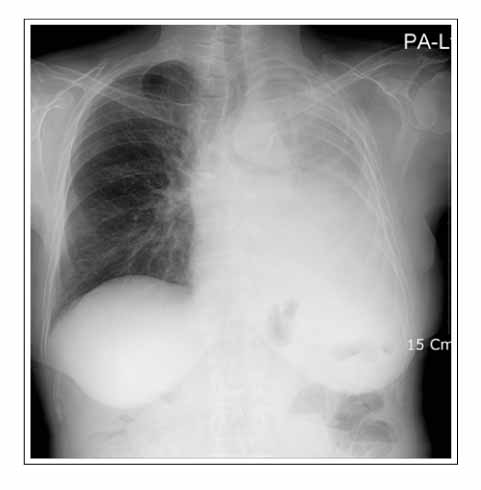
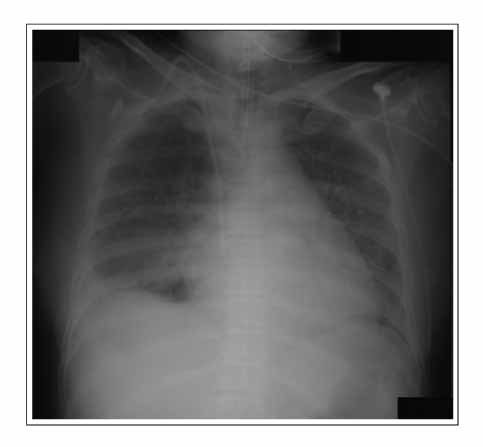
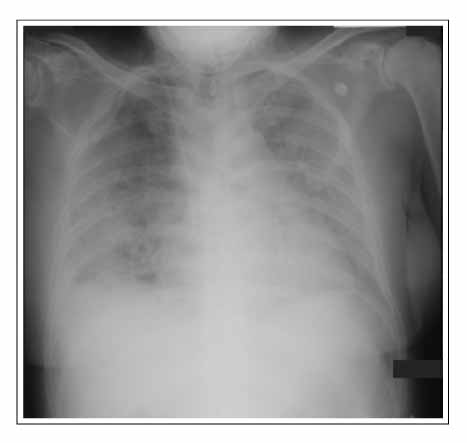
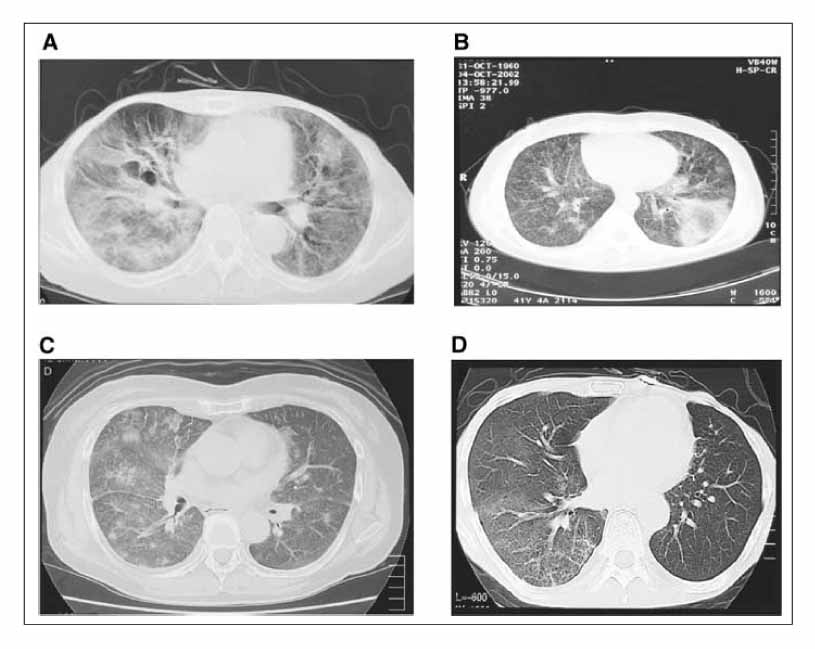
- Gefitinib is a novel drug used to treat advanced non-small cell lung cancer. However, drug-related interstitial pneumonia is a major life-threatening side effect, which has a worldwide prevalence of 0.3-0.4%. In Japan, the prevalence is high as 3-4% but the actual frequency in Korea has not been officially assessed. We report two cases of gefitinib-induced interstitial lung disease during the treatment of non-small cell lung cancer. High-resolution computerized tomography (HRCT) of one case showed nonspecific ground glass opacity and the chest x-ray of another case showed diffuse bilateral ground glass opacity. The former patient showed a rapid good response to corticosteroid treatment whereas the latter died despite receiving aggressive treatment with high dose corticosteroid and empirical antibiotics.
MeSH Terms
Figure
Reference
-
1. Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001. 13:491–498.2. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001. 7:2958–2970.3. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003. 21:2237–2246.4. Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003. 361:137–139.5. Forsythe B, Faulkner K. Safety and tolerability of gefitinib ('Iressa', ZD1839) in advanced NSCLC: overview of clinical experience. 2003b. In : Presentation at the ERS; September 27-October 1; Vienna, Austria. P327.6. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.7. Kang JH, Kang SY, Kim HT, Lee JS, Shim BY, Park BB, et al. Retrospective Analysis of Patients with Advanced Non-Small Cell Lung Cancer Who Participated in the Extended Access Program (EAP) of Gefitinib. In : Presentation at the CELCC; June 18-21; Praque, Czech Republic. –O20.8. Iressa Expert Committee. Expert Committee Meeting Report. Final report on interstitial lung disease (ILD) related to gefitinib (Iressa Tablet 250). 2003. March 26; AstraZeneca.9. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004. 91:S18–S23.10. Muller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004. 91:S24–S30.11. Endo M, Johkoh T, Kimura K, Yamamoto N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006. 52:135–140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Idiopathic Interstitial Pneumonias: Radiologic Findings
- A Case of Nonspecific Interstitial Pneumonia with Clinical Course of Rapid Aggravation
- Effect of interferon-gamma treatment on interstitial pneumonia in a patient with severe combined immunodeficiency
- Idiopathic interstitial pneumonias: clinical findings, pathogenesis, pathology and radiologic findings
- Gefitinib-Induced Interstitial Lung Disease in Korean Lung Cancer Patients