J Korean Soc Hypertens.
2011 Mar;17(1):17-27. 10.5646/jksh.2011.17.1.17.
Effects of Angiotensin II and Shear Stress Interaction on Vascular Inflammation
- Affiliations
-
- 1Laboratory of Cardiovascular Research, The Catholic University of Korea School of Medicine, Seoul, Korea. whitesh@catholic.ac.kr
- 2Division of Cardiovascular Medicine, St. Seoul Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 3Department Biology, University of Miami College of Arts and Sciences,Coral Gables, FL, USA.
- KMID: 2099898
- DOI: http://doi.org/10.5646/jksh.2011.17.1.17
Abstract
- BACKGROUND
Angiotensin II (AngII) and abnormal oscillatory shear stress are highly associated with vascular inflammation including atherosclerosis. However, it is poorly understood how interactions between AngII and shear stress in human aortic endothelial cells (HAEC) are involved in mechanisms by which cellular adhesion molecules are expressed. The purpose of this study was to improve that understanding.
METHODS
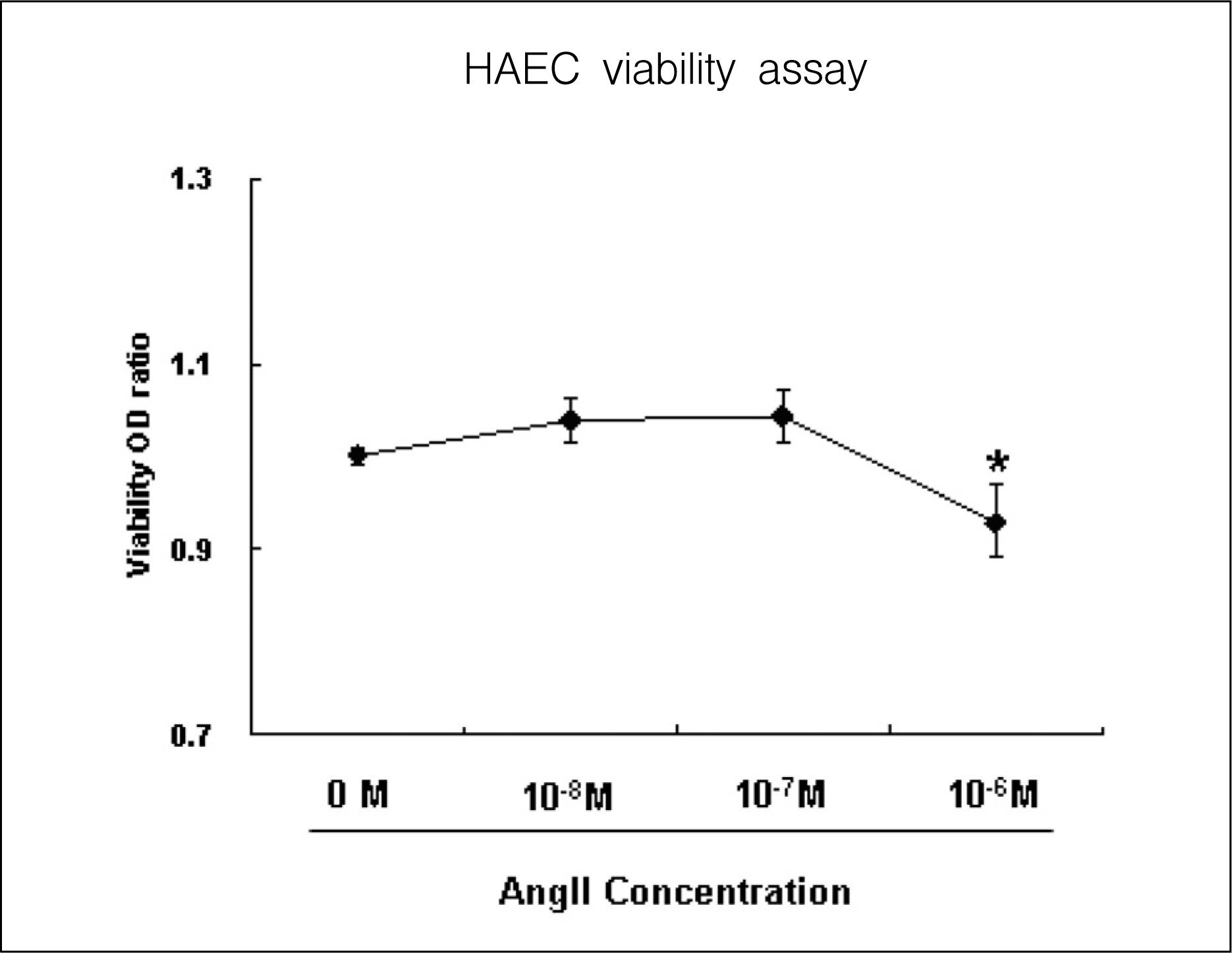
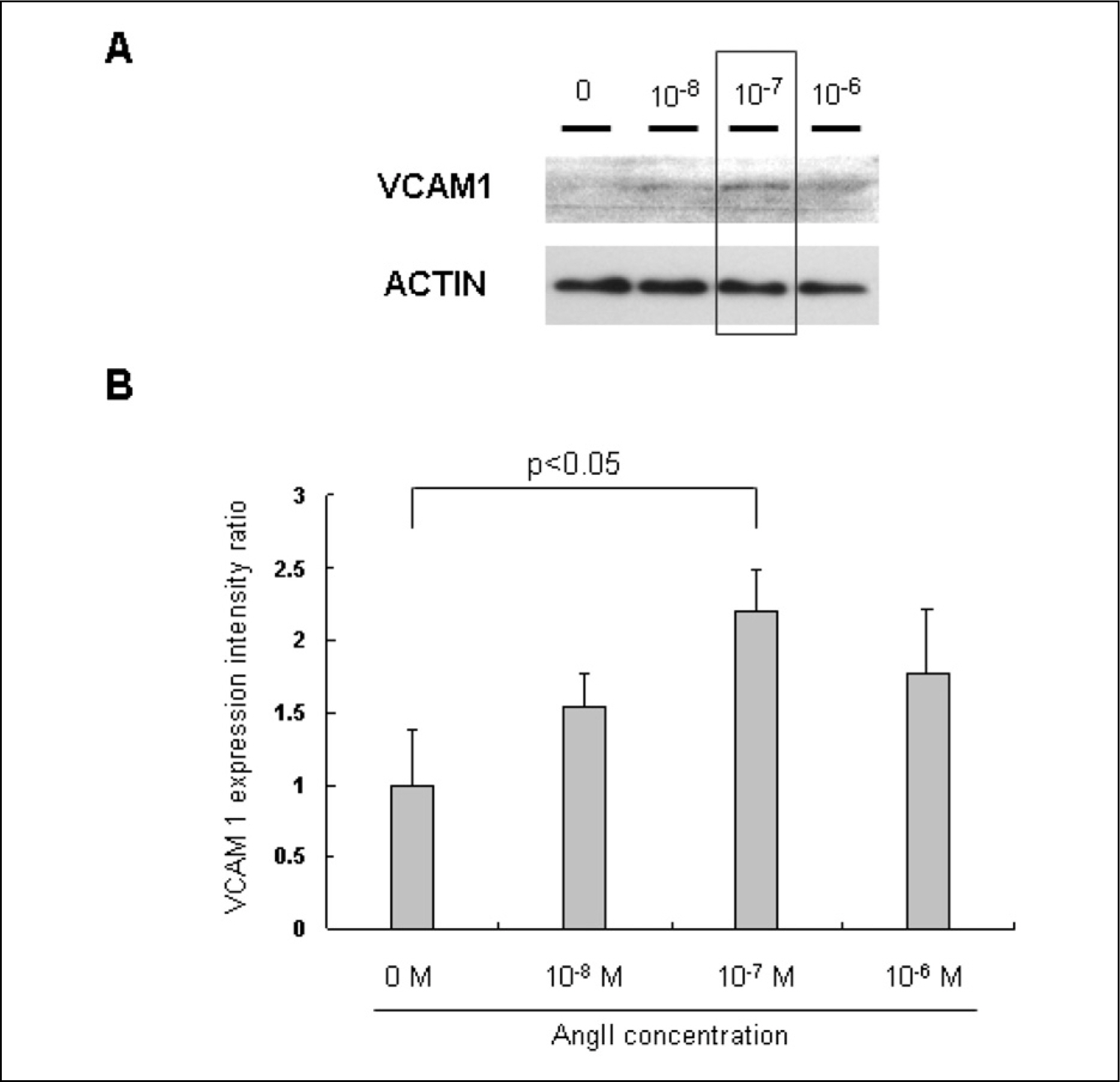
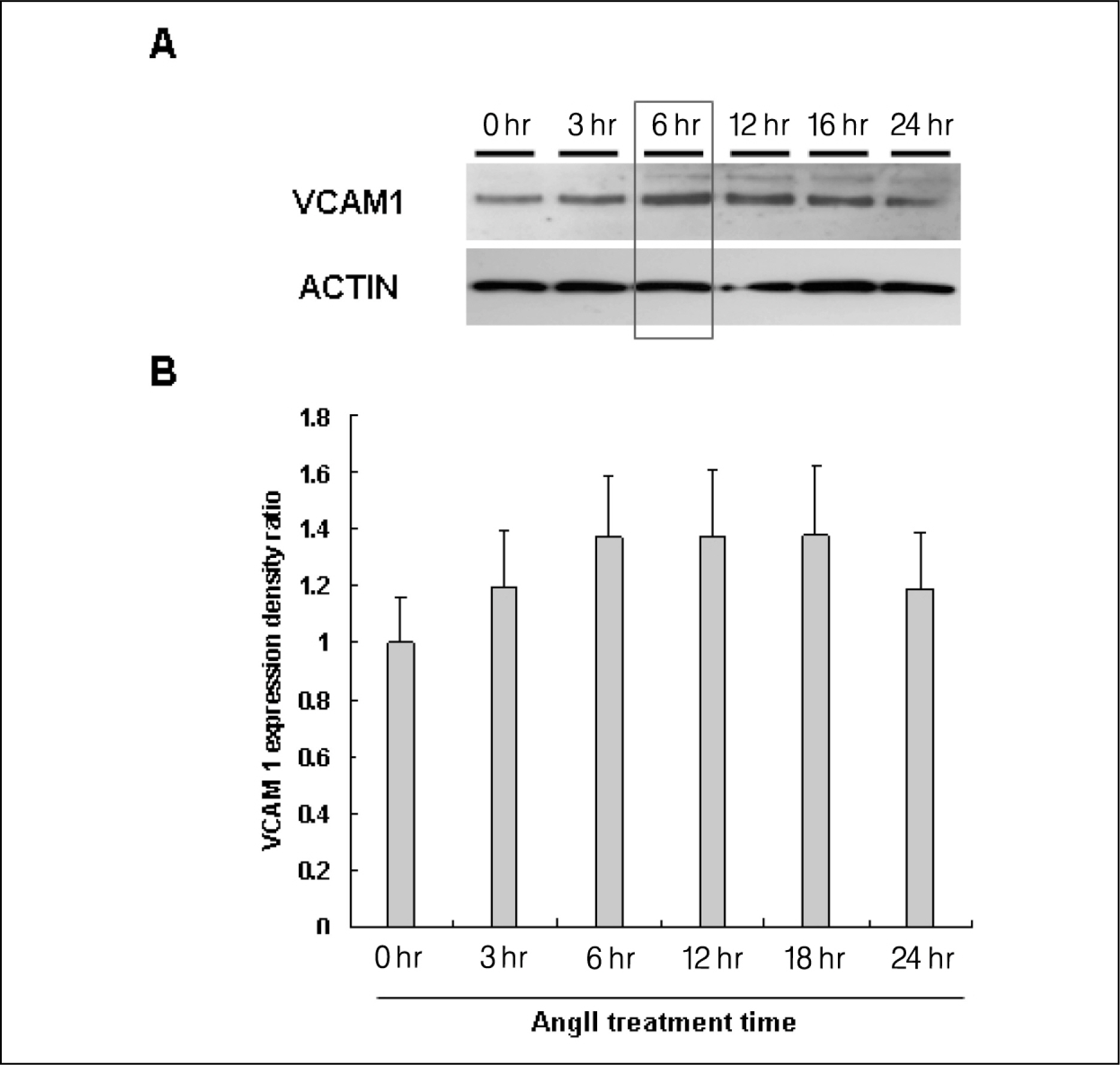
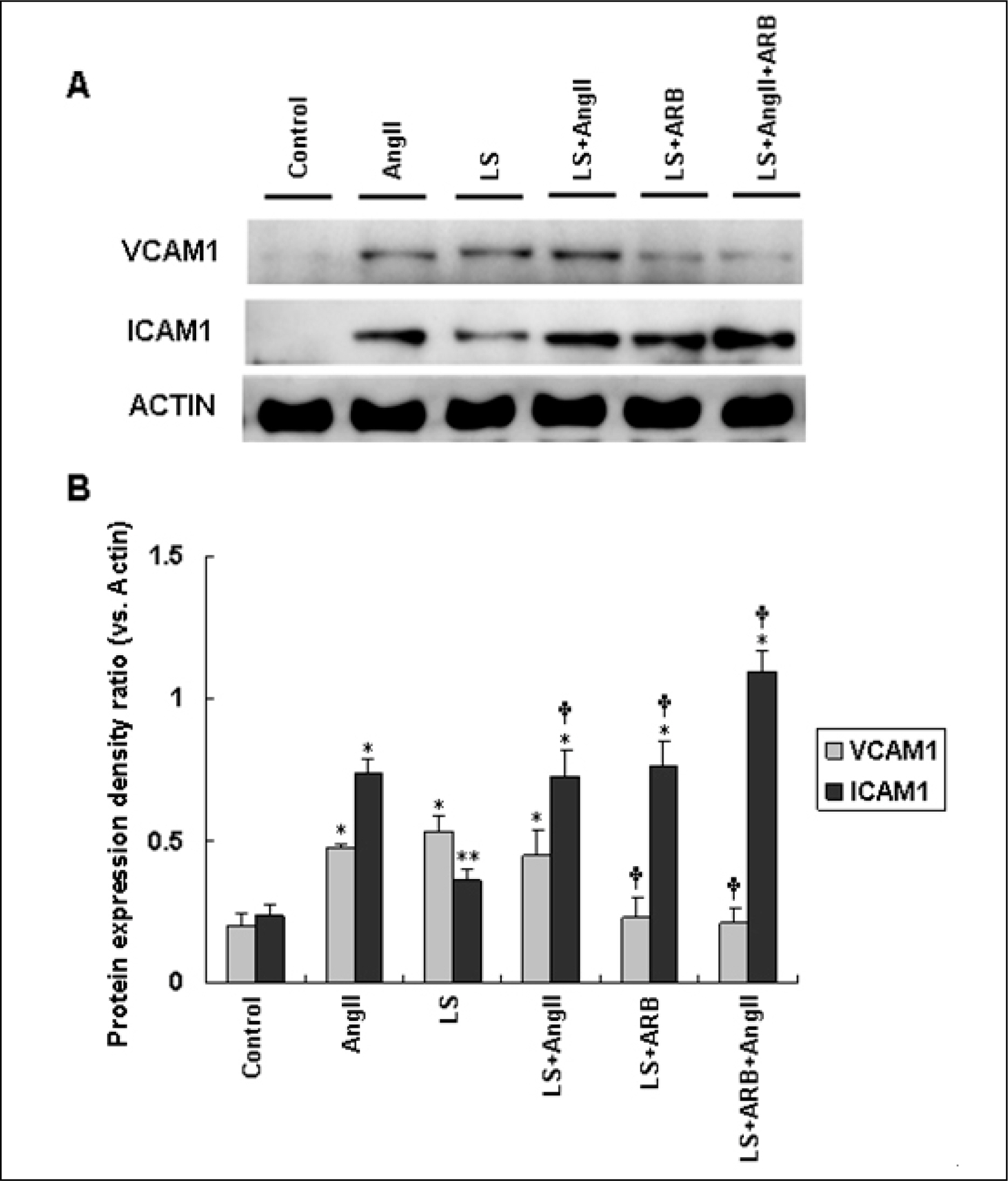
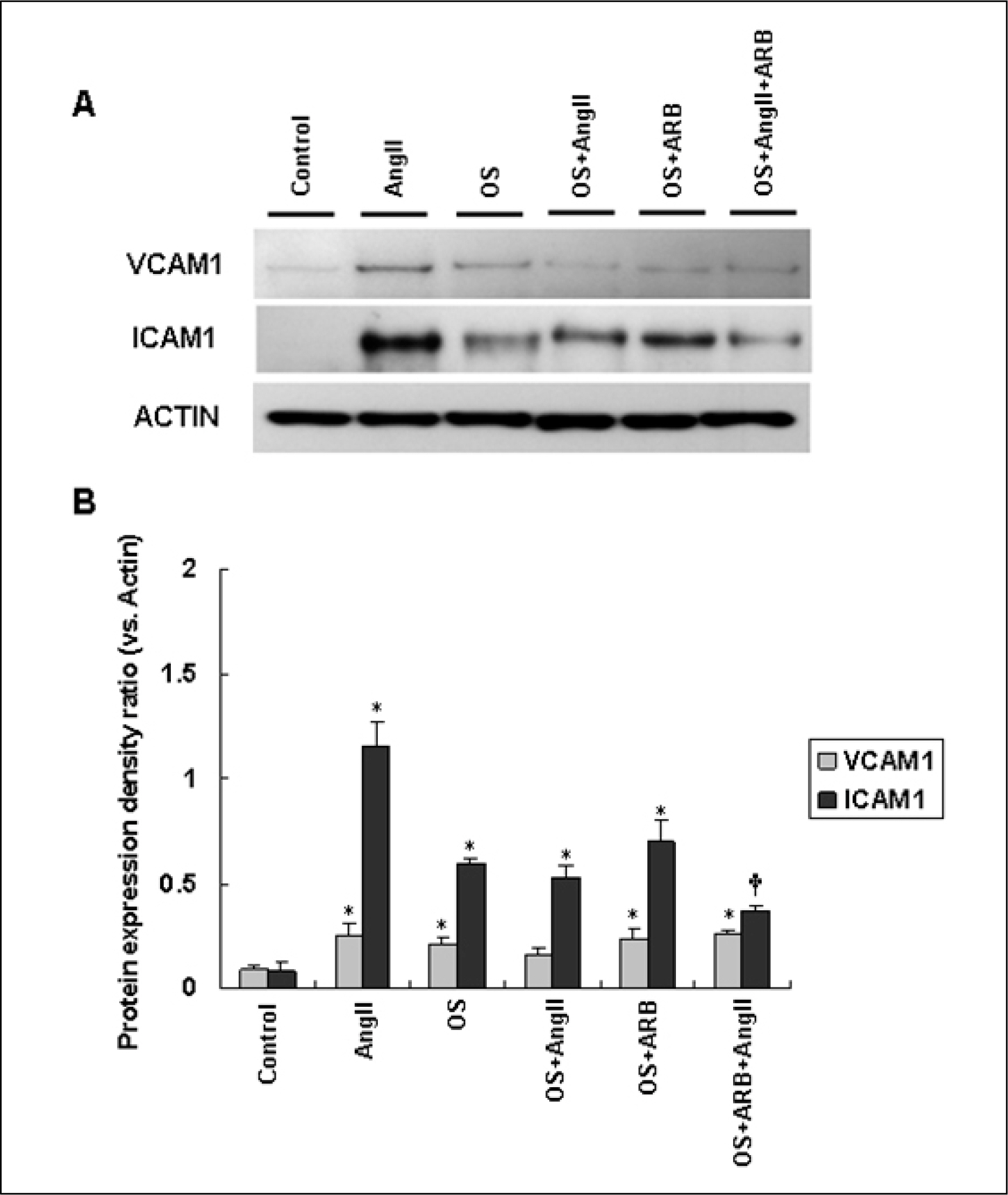
AngII (10(-7)M for 6 hr) and two-types of shear stress treatments were used: laminar shear stress (LS: unidirectional, 12 dynes/cm2) and oscillatory shear stress (OS: bi-directional, 5 dynes/cm2, 1 Hz) in HAEC. Immunoblotting was used to detect expression of cellular adhesion molecules markers such as vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1).
RESULTS
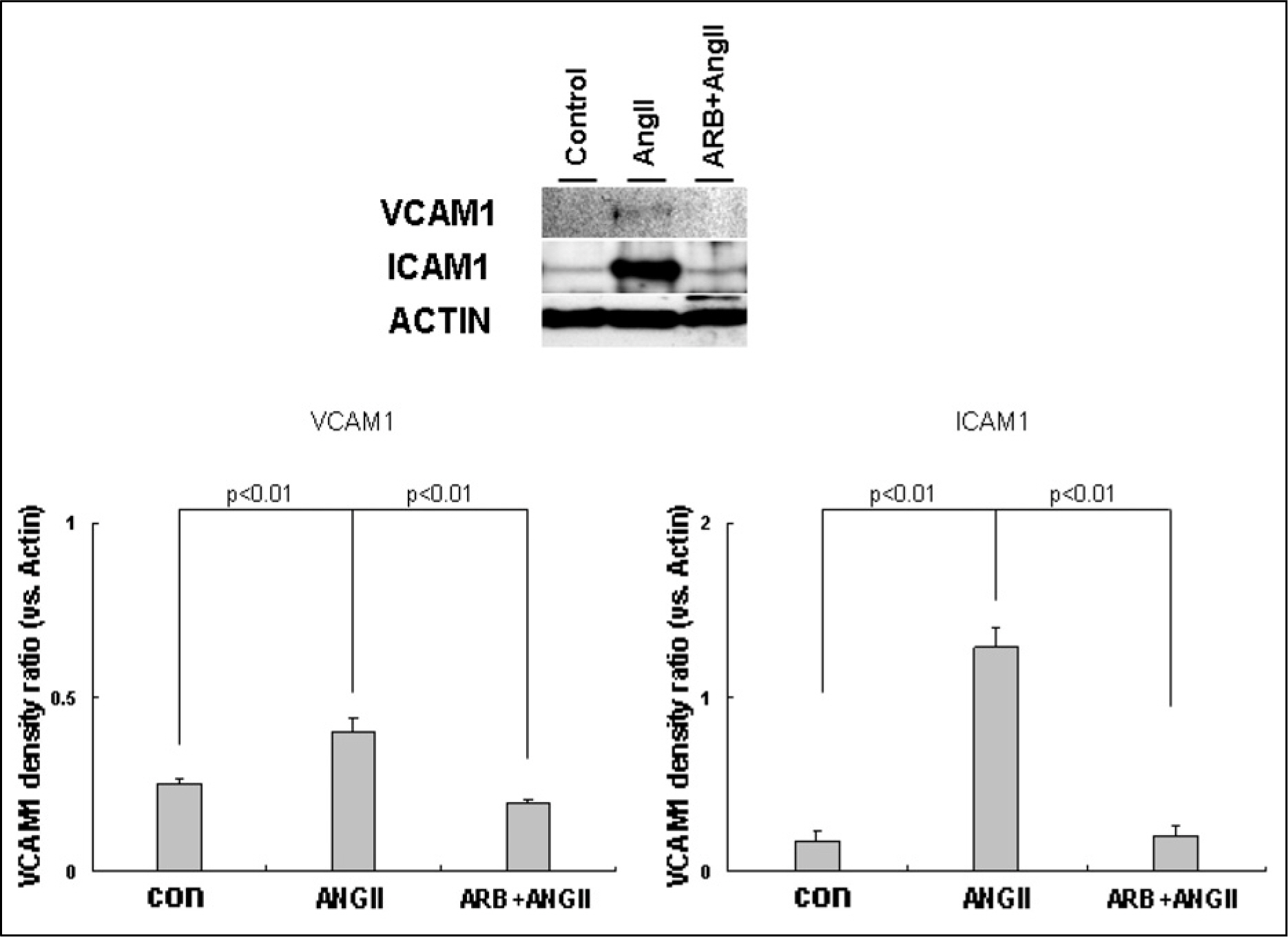
AngII significantly increased VCAM1 and ICAM1 expression in HAEC that had been reduced due to pretreatment with telmisartan. AngII-LS co-stimulation and AngII-OS co-stimulation significantly increased VCAM1 and ICAM1 expression in HAEC. The expression levels of VCAM1 and ICAM1 were also, significantly reduced when pretreated with telmisartan. However, VCAM1 and ICAM1 expression were significantly reduced under LS and OS stimulation.
CONCLUSIONS
Telmisartan may modulate the expressions of VCAM1 and ICAM1 via different types of shear stress in HAEC that are activated by AngII.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105:1135–43.
Article2. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994; 74:1141–8.
Article3. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001; 37:1053–9.4. Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008; 102:201–8.
Article5. Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003; 79:195–9.
Article6. Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993; 171:223–9.
Article7. Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998; 18:842–51.
Article8. Ando J, Tsuboi H, Korenaga R, Takada Y, Toyama-Sorimachi N, Miyasaka M, et al. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by downregulating VCAM-1 expression. Am J Physiol. 1994; 267:C679–87.
Article9. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999; 282:2035–42.
Article10. Catt KJ, Mendelsohn FA, Millan MA, Aguilera G. The role of angiotensin II receptors in vascular regulation. J Cardiovasc Pharmacol. 1984; 6(Suppl 4):S575–86.
Article11. Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007; 22:311–5.
Article12. Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008; 29:367–74.
Article13. Choi SH, Park EH, Sim CE, Baek SH. Effects of angiotensin II receptor blockers on endothelial progenitor cells in spontaneously hypertensive rats. J Korean Soc Hypertens. 2009; 15:42–50.14. Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000; 20:645–51.15. Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, et al. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999; 100:1223–9.16. Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995; 15:2–10.
Article17. Weiss D, Sorescu D, Taylor WR. Angiotensin II and atherosclerosis. Am J Cardiol. 2001; 87(Suppl):25C–32C.
Article18. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005; 85:9–23.
Article19. Go YM, Boo YC, Park H, Maland MC, Patel R. Pritchard KA Jr, et al. Protein kinase B/Akt activates c-Jun NH(2)-terminal kinase by increasing NO production in response to shear stress. J Appl Physiol. 2001; 91:1574–81.20. Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003; 278:31128–35.
Article21. Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Pept. 2000; 93:65–77.
Article22. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995; 91:2488–96.23. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999; 340:115–26.24. Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002; 106:820–5.
Article25. Berry C, Brosnan MJ, Fennell J, Hamilton CA, Domi-niczak AF. Oxidative stress and vascular damage in hypertension. Curr Opin Nephrol Hypertens. 2001; 10:247–55.
Article26. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hyper-tension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996; 97:1916–23.
Article27. Abe J, Berk BC. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc Med. 1998; 8:59–64.
Article28. Charles RL, Eaton P. Redox signalling in cardiovascular disease. Proteomics Clin Appl. 2008; 2:823–36.
Article29. Fukuda D, Enomoto S, Hirata Y, Nagai R, Sata M. The angiotensin receptor blocker, telmisartan, reduces and stabilizes atherosclerosis in ApoE and AT1aR double deficient mice. Biomed Pharmacother. 2010; 64:712–7.
Article30. An J, Nakajima T, Kuba K, Kimura A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens Res. 2010; 33:831–5.31. Yao R, Cheng X, Chen Y, Xie JJ, Yu X, Liao MY, et al. Molecular mechanisms of irbesartan suppressing atherosclerosis in high cholesterol-diet apolipoprotein E knock-out mice. Int J Cardiol. 2010; 139:113–22.32. O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993; 92:945–51.33. O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996; 93:672–82.
Article34. Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998; 18:677–85.35. Chiu JJ, Lee PL, Chen CN, Lee CI, Chang SF, Chen LJ, et al. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol. 2004; 24:73–9.36. Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregu-lation of endothelial VCAM-1 and ICAM-1 in a BMP-4-and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009; 29:254–60.37. Mohan S, Mohan N, Valente AJ, Sprague EA. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am J Physiol. 1999; 276:C1100–7.
Article38. Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994; 201:950–6.39. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994; 12:141–79.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overview of the Renin-Angiotensin System
- The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications
- Epigallocatechin-3-gallate Regulates Inducible Nitric Oxide Synthase Expression in Human Umbilical Vein Endothelial Cells
- Effects of Angiotensin II on the Growth of Vascular Smooth Muscle Cells
- Renin Angiotensin System in Rabbit Corpus Cavernosum: Functional Characterization of Angiotensin II Receptors