J Korean Soc Magn Reson Med.
2012 Aug;16(2):124-135. 10.13104/jksmrm.2012.16.2.124.
Structural and Functional Changes of Hippocampus in Long Life Experienced Taxi Driver
- Affiliations
-
- 1Department of Radiology, Graduate School of Medicine, Kyung Hee University, Korea.
- 2Computational NeuroImage Analysis Lab, Department of Biomedical Engineering, Hanyang University, Korea.
- 3Department of Radiology, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Korea. ghjahng@gmail.com
- 4Department of Radiology, Kyung Hee University Hospital, School of Medicine, Kyung Hee University, Korea.
- KMID: 2099846
- DOI: http://doi.org/10.13104/jksmrm.2012.16.2.124
Abstract
- PURPOSE
The objective of this study was to investigate the differences of hippocampal volume and shape as well as the functional change between long life experienced taxi drivers and controls of Korean population.
MATERIALS AND METHODS
Three-dimensional T1-weighted images and blood oxygen level dependent functional MRI(fMRI) were obtained from 8 subjects, consisting of 4 experienced (20-30 years) taxi drivers and 4 age-matched controls. The hippocampal volume and shape were analyzed with three-dimensional T1-weighted images. In addition, neuronal activities of brain were analyzed using a blood oxygen level dependent fMRI between the two groups.
RESULTS
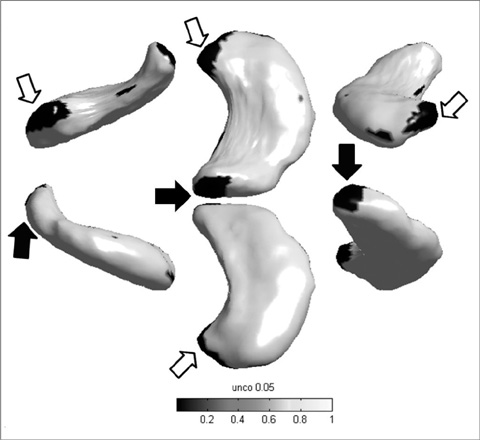
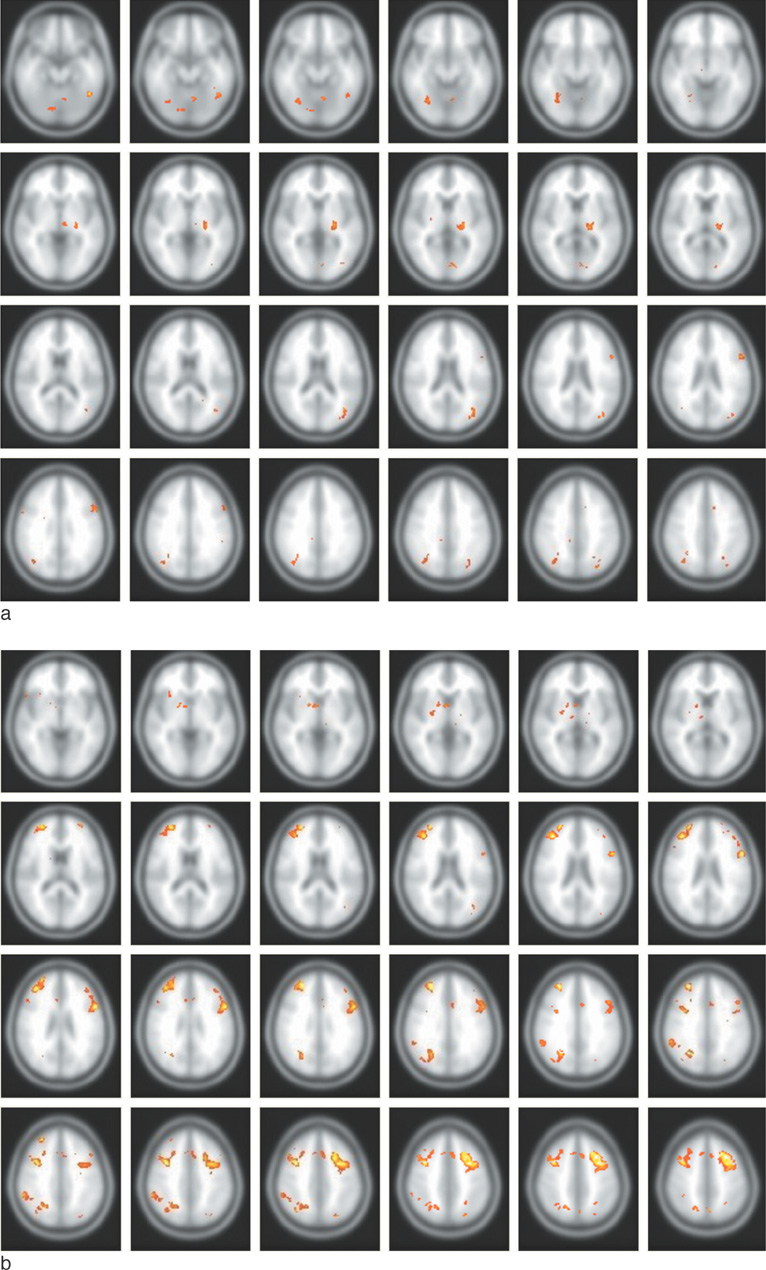
The hippocampal volume showed no statistically significant difference between the two groups (p > 0.05). The left hippocampi of the taxi drivers were slightly elongated with larger head and tail portions than those of the controls (p < 0.05, uncorrected). For the functional MRI, fusiform gyrus was specifically activated in taxi drivers, compared with the control group.
CONCLUSION
The structural and functional changes of taxi driver's hippocampus indicate the functional differentiation as a result of occupational dependence on spatial navigation. In other words, the continuous usage of spatial navigation performance may diminish degeneration of hippocampus and the related brain regions.
Figure
Reference
-
1. Kakeda S, Korogi Y. The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and alzheimer's disease/mild cognitive impairment: a review. Neuroradiology. 2010. 52:711–721.2. Tae WS, Kim SS, Lee KU, Nam EC, Choi JW, Park JI. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011. 32:671–676.3. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990. 87:9868–9872.4. Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional mri in adhd: a systematic literature review. Expert Rev Neurother. 2007. 7:1337–1356.5. Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007. 45:174–194.6. Weiss EM, Siedentopf C, Golaszewski S, et al. Brain activation patterns during a selective attention test--a functional mri study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007. 154:31–40.7. Chen G, Ward BD, Xie C, et al. Classification of alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology. 2011. 259:213–221.8. Slotnick SD. Does the hippocampus mediate objective binding or subjective remembering? Neuroimage. 2010. 49:1769–1776.9. Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000. 97:4398–4403.10. Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006. 31:1826–1840.11. Kumaran D, Maguire EA. The human hippocampus: cognitive maps or relational memory? J Neurosci. 2005. 25:7254–7259.12. Firbank MJ, Barber R, Burton EJ, O'Brien JT. Validation of a fully automated hippocampal segmentation method on patients with dementia. Hum Brain Mapp. 2008. 29:1442–1449.13. Lee JM, Kim SH, Jang DP, et al. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. Neuroimage. 2004. 22:831–840.14. Chung MK, Worsley KJ, Robbins S, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003. 18:198–213.15. Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the balloon model, volterra kernels, and other hemodynamics. Neuroimage. 2000. 12:466–477.16. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000. 11:805–821.17. Talairach J, Szikla G. Application of stereotactic concepts to the surgery of epilepsy. Acta Neurochir Suppl (Wien). 1980. 30:35–54.18. Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002. 17:1365–1372.19. Woollett K, Maguire EA. Navigational expertise may compromise anterograde associative memory. Neuropsychologia. 2009. 47:1088–1095.20. de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with alzheimer's disease. Hippocampus. 2000. 10:136–142.21. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000. 157:115–118.22. Woollett K, Spiers HJ, Maguire EA. Talent in the taxi: a model system for exploring expertise. Philos Trans R Soc Lond B Biol Sci. 2009. 364:1407–1416.23. Mion M, Patterson K, Acosta-Cabronero J, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010. 133:3256–3268.24. Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006. 16:716–729.25. Rosenbaum RS, Gao F, Richards B, Black SE, Moscovitch M. "Where to?" Remote memory for spatial relations and landmark identity in former taxi drivers with alzheimer's disease and encephalitis. J Cogn Neurosci. 2005. 17:446–462.26. Maguire EA, Nannery R, Spiers HJ. Navigation around london by a taxi driver with bilateral hippocampal lesions. Brain. 2006. 129:2894–2907.27. Banquet JP, Gaussier P, Quoy M, Revel A, Burnod Y. A hierarchy of associations in hippocampo-cortical systems: cognitive maps and navigation strategies. Neural Comput. 2005. 17:1339–1384.28. Burgess N, O'Keefe J. Neuronal computations underlying the firing of place cells and their role in navigation. Hippocampus. 1996. 6:749–762.29. Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001. 42:225–238.30. Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000. 3:404–408.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study for the Development of Prostate Associated Urinary Tract Symptoms in Occupational Taxi Drivers
- Blood Lead Concentration of Taxi Drivers in Taegu, Korea
- Respiratory Symptoms and Ventilatory Function Impairment of Taxi Drivers
- The Effects of Customer Contact Service to the Mental Health among Korean Taxi Drivers
- Perceptions of the Risk of Cardiovascular Disease in Middle-aged Male Taxi Drivers: Focus Group Interviews