Korean Circ J.
2012 Jul;42(7):458-463. 10.4070/kcj.2012.42.7.458.
A Comparison of Two Brands of Clopidogrel in Patients With Drug-Eluting Stent Implantation
- Affiliations
-
- 1Cardiology Division, Department of Internal Medicine, Gil Hospital, Gachon University of Medicine and Science, Incheon, Korea. encore@gilhospital.com
- KMID: 2094117
- DOI: http://doi.org/10.4070/kcj.2012.42.7.458
Abstract
- BACKGROUND AND OBJECTIVES
Although generic clopidogrel is widely used, clinical efficacy and safety between generic and original clopidogrel had not been well evaluated. The aim of this study was to evaluate the clinical outcomes of 2 oral formulations of clopidogrel 75 mg tablets in patients with coronary artery disease (CAD) undergoing drug-eluting stent (DES) implantation.
SUBJECTS AND METHODS
Between July 2006 and February 2009, 428 patients that underwent implantation with DES for CAD and completed >1 year of clinical follow-up were enrolled in this study. Patients were divided into the following 2 groups based on treatment formulation, Platless(R) (test formulation, n=211) or Plavix(R) (reference formulation, n=217). The incidence of 1-year major adverse cardiovascular and cerebrovascular event (MACCE) and stent thrombosis (ST) were retrospectively reviewed.
RESULTS
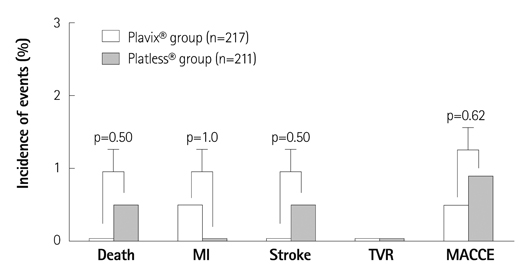
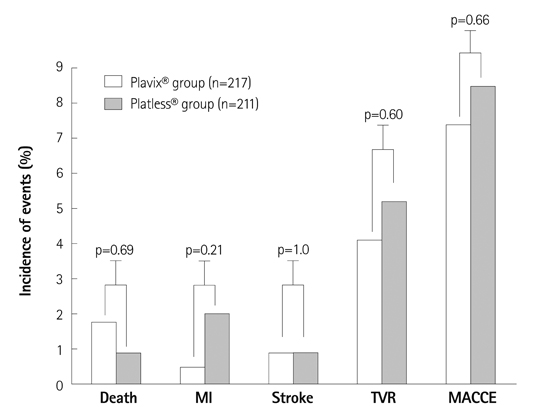
The baseline demographic and procedural characteristics were not significantly different between two treatment groups. The incidence of 1-year MACCEs was 8.5% {19/211, 2 deaths, 4 myocardial infarctions (MIs), 2 strokes, and 11 target vessel revascularizations (TVRs)} in Platless(R) group vs. 7.4% (16/217, 4 deaths, 1 MI, 2 strokes, and 9 TVRs) in Plavix(R) group (p=0.66). The incidence of 1-year ST was 0.5% (1 definite and subacute ST) in Platless(R) group vs. 0% in Plavix(R) group (p=0.49).
CONCLUSION
In this study, the 2 tablet preparations of clopidogrel showed similar rates of MACCEs, but additional prospective randomized studies with pharmacodynamics and platelet reactivity are needed to conclude whether generic clopidgrel may replace original clopidogrel.
MeSH Terms
Figure
Reference
-
1. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001. 345:494–502.2. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001. 358:527–533.3. Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002. 288:2411–2420.4. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006. 113:2363–2372.5. Wood S. HeartWire: mixed thoughts on how generic clopidogrel might impact patients, providers [Internet]. 2006. cited 2010 Mar 15. Montreal: theheart.org;Available from: http://www.theheart.org/article/729929.do.6. Gomez Y, Adams E, Hoogmartens J. Analysis of purity in 19 drug product tablets containing clopidogrel: 18 copies versus the original brand. J Pharm Biomed Anal. 2004. 34:341–348.7. Kim SD, Kang W, Lee HW, et al. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther. 2009. 31:793–803.8. Bahrami G, Mohammadi B, Sisakhtnezhad S. High-performance liquid chromatographic determination of inactive carboxylic acid metabolite of clopidogrel in human serum: application to a bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. 864:168–172.9. Colombo A, Orlic D, Stankovic G, et al. Preliminary observations regarding angiographic pattern of restenosis after rapamycin-eluting stent implantation. Circulation. 2003. 107:2178–2180.10. Applegate RJ, Grabarczyk MA, Little WC, et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol. 2002. 40:78–83.11. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007. 115:2344–2351.12. Di Girolamo G, Czerniuk P, Bertuola R, Keller GA. Bioequivalence of two tablet formulations of clopidogrel in healthy Argentinian volunteers: a single-dose, randomized-sequence, open-label crossover study. Clin Ther. 2010. 32:161–170.13. Jeong YH, Koh JS, Kang MK, et al. The impact of generic clopidogrel bisulfate on platelet inhibition in patients with coronary artery stents: results of the ACCEL-GENERIC study. Korean J Intern Med. 2010. 25:154–161.14. Robinson A, Hillis J, Neal C, Leary AC. The validation of a bioanalytical method for the determination of clopidogrel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. 848:344–354.15. From AM, Al Badarin FJ, Cha SS, Rihal CS. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis of data from the ARTS II, CARDia, ERACI III, and SYNTAX studies and systematic review of observational data. EuroIntervention. 2010. 6:269–276.16. Akin I, Bufe A, Schneider S, et al. Clinical outcomes in diabetic and non-diabetic patients with drug-eluting stents: results from the first phase of the prospective multicenter German DES.DE registry. Clin Res Cardiol. 2010. 99:393–400.17. Suh J, Park DW, Lee JY, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc Interv. 2010. 3:383–389.18. Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diameter of drug-eluting stents versus bare-metal stents on late outcomes. Circ Cardiovasc Interv. 2009. 2:35–42.19. Weerakkody GJ, Brandt JT, Payne CD, Jakubowski JA, Naganuma H, Winters KJ. Clopidogrel poor responders: an objective definition based on Bayesian classification. Platelets. 2007. 18:428–435.20. Barragan P, Bouvier JL, Roquebert PO, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003. 59:295–302.21. Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003. 89:783–787.22. Katoh M, Nakajima M, Shimada N, Yamazaki H, Yokoi T. Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000. 55:843–852.23. Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008. 52:1557–1563.24. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart. 2010. 96:186–189.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ticagrelor Rescue Therapy in a Patient with Subacute Stent Thrombosis
- A Case of Stent Thrombosis Occurred at 5 Years after Sirolimus-Eluting Stent Implantation
- Successful Prasugrel Rescue Therapy in Clopidogrel Resistant Patients Who Had Recurrent Stent Thrombosis of Drug-Eluting-Stent: The Role of Prasugrel in Clopidogrel Nonresponders
- Systemic drug therapy and restenosis after drug-eluting stent implantation
- Very Late Stent Thrombosis after Drug-Eluting Stent Implantation in a Patient without Aspirin and Clopidogrel Resistance