Korean J Hematol.
2007 Jun;42(2):167-171. 10.5045/kjh.2007.42.2.167.
Quantifing the Circulating Epstein-Barr Virus (EBV) DNA to Monitor a Case of Aggressive Natural Killer Cell Leukemia
- Affiliations
-
- 1Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wskimsmc@smc.samsung.co.kr
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2083516
- DOI: http://doi.org/10.5045/kjh.2007.42.2.167
Abstract
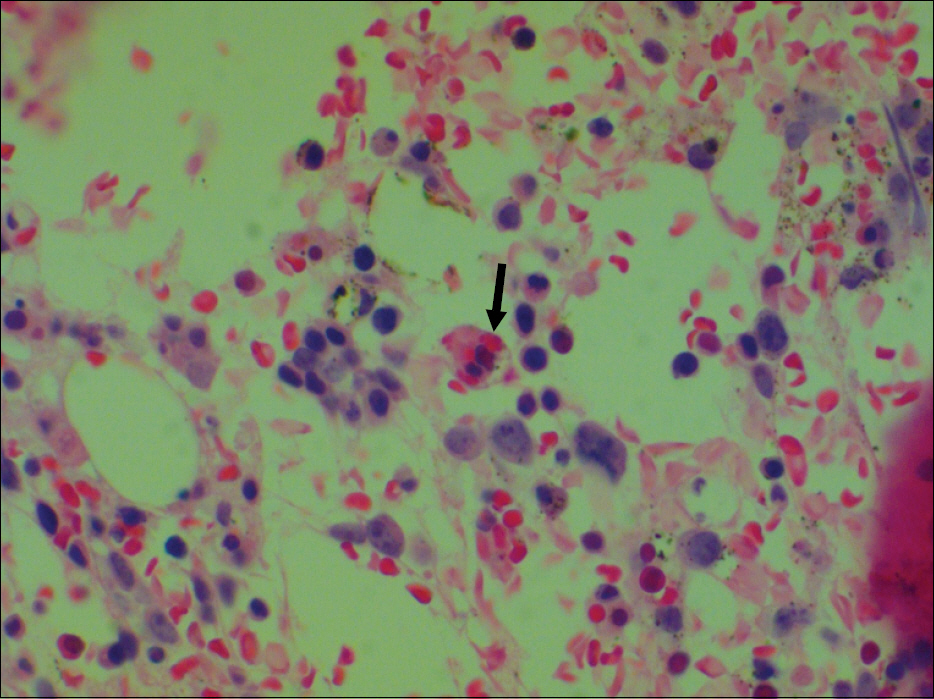
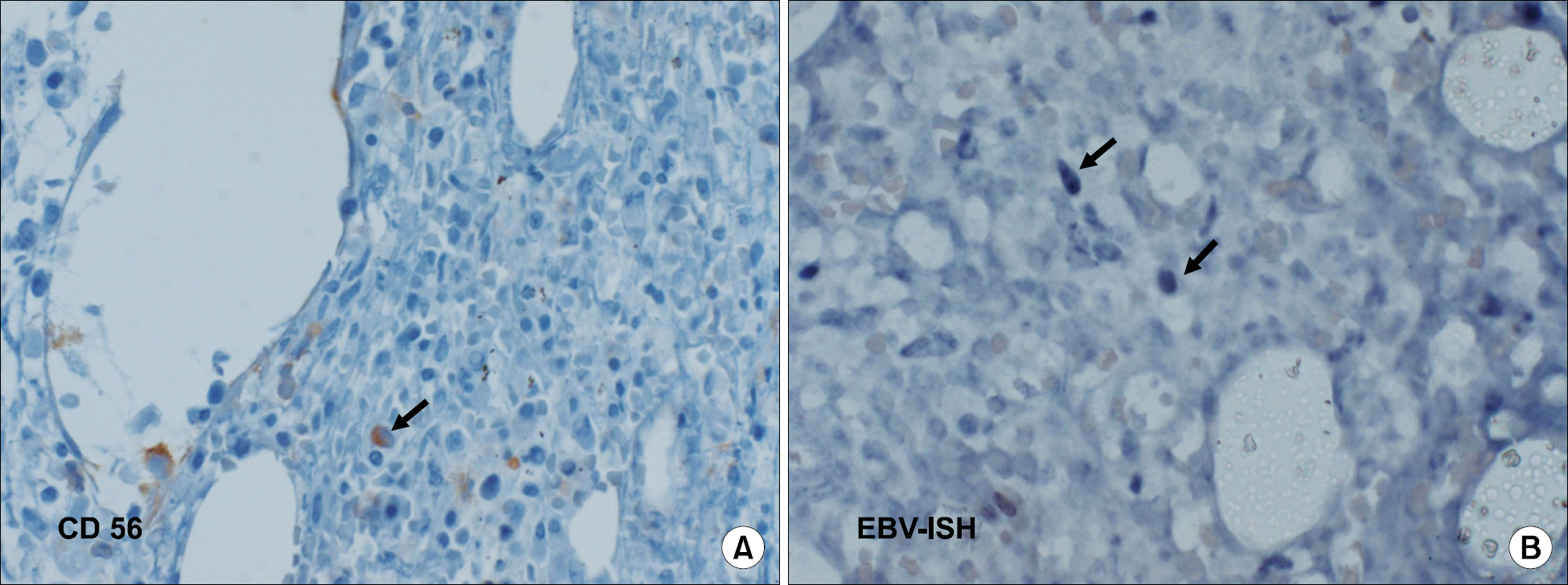
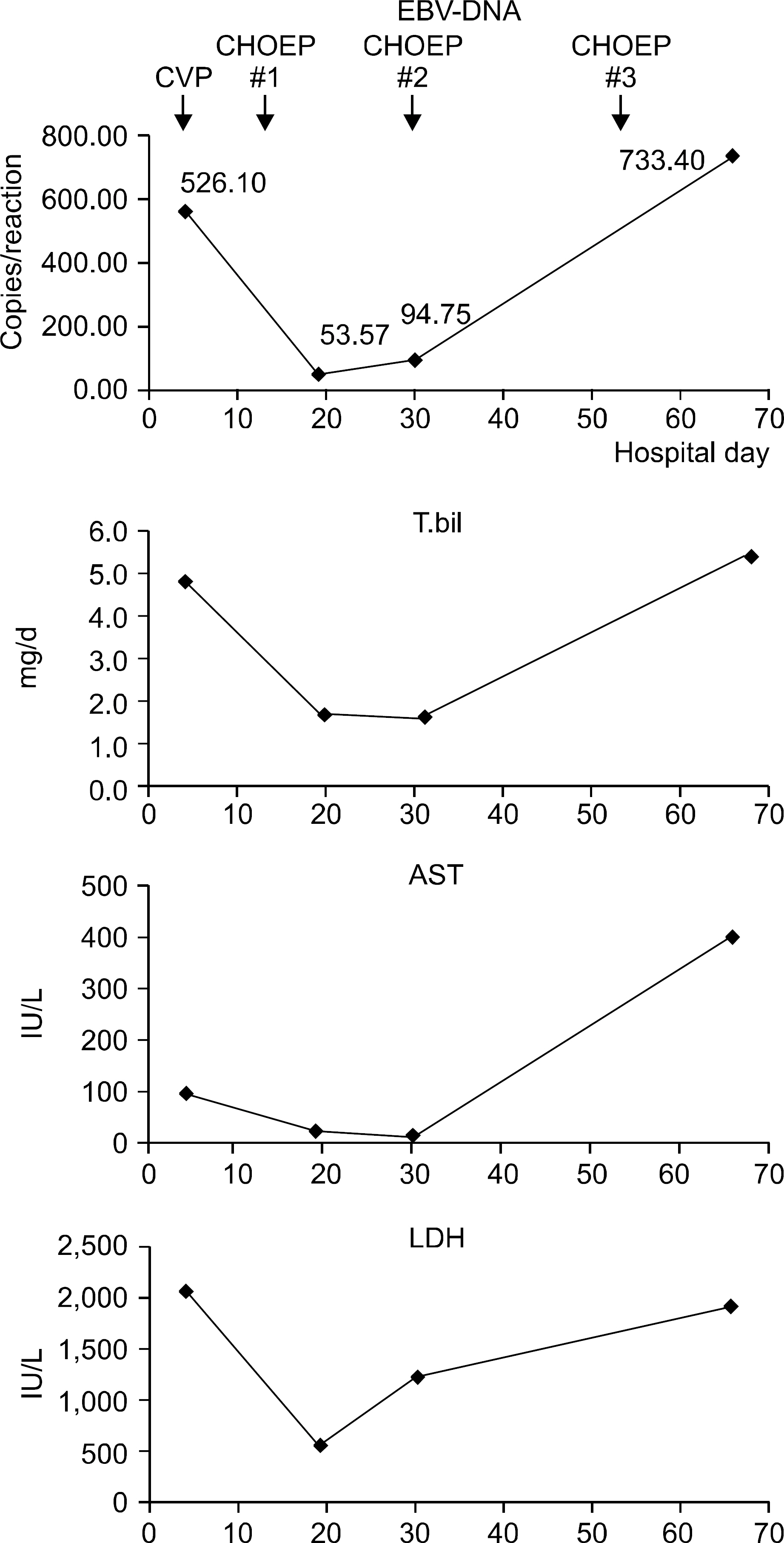
- Epstein-Barr virus (EBV) infection is associated with various lymphoproliferative diseases, including Hodgkin lymphoma, extranodal NK/T cell lymphoma, aggressive NK cell leukemia, Burkitt lymphoma and post-transplant lymphoproliferative disorder. In the recent studies, the plasma EBV-DNA levels in patients with EBV-associated lymphoproliferative disease appeared to be correlated with the therapeutic response. Aggressive NK cell leukemia (ANKL) is a fatal disease that's characterized by high fever, lymphadenopathy, hepatosplenomegaly and frequent hemophagocytosis. No serological tumor marker for this malignancy has yet been identified for monitoring the disease and predicting the outcome. We experienced a case of aggressive natural killer cell leukemia in a 48-year-old man, and we serially monitored the plasma EBV DNA load by performing real time quantitative PCR assay. Serial measurements of the plasma EBV DNA level during therapy showed a close correlation between the clinical response and the changes in the plasma EBV DNA titers.
Keyword
MeSH Terms
Figure
Reference
-
1). Gulley ML. Molecular diagnosis of Epstein-Barr virus-related diseases. J Mol Diagn. 2001. 3:1–10.
Article2). Au WY., Pang A., Choy C., Chim CS., Kwong YL. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004. 104:243–9.
Article3). Lei KI., Chan LY., Chan WY., Johnson PJ., Lo YM. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin Cancer Res. 2002. 8:29–34.4). Lei KI., Chan LY., Chan WY., Johnson PJ., Lo YM. Quantitative analysis of circulating cell-free Epstein-Barr virus (EBV) DNA levels in patients with EBV-associated lymphoid malignancies. Br J Haematol. 2000. 111:239–46.
Article5). Ruskova A., Thula R., Chan G. Aggressive natural killer-cell leukemia: report of five cases and review of the literature. Leuk Lymphoma. 2004. 45:2427–38.
Article6). Murdock J., Jaffe ES., Wilson WH., McManus DT., Alexander HD., Morris TC. Aggressive natural killer cell leukemia/lymphoma: case report, use of telesy-nergy and review of the literature. Leuk Lymphoma. 2004. 45:1269–73.
Article7). Song SY., Kim WS., Ko YH., Kim K., Lee MH., Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002. 87:1343–5.8). Imamura N., Kusunoki Y., Kawa-Ha K, et al. Aggressive natural killer cell leukaemia/lymphoma: report of four cases and review of the literature. Possible existence of a new clinical entity originating from the third lineage of lymphoid cells. Br J Haematol. 1990. 75:49–59.
Article9). Suzuki R., Suzumiya J., Nakamura S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004. 18:763–70.
Article10). Mori N., Yamashita Y., Tsuzuki T, et al. Lymphomatous features of aggressive NK cell leukaemia/lymphoma with massive necrosis, haemophagocytosis and EB virus infection. Histopathology. 2000. 37:363–71.
Article11). Kwong YL., Chan AC., Liang RH. Natural killer cell lymphoma/leukemia: pathology and treatment. Hematol Oncol. 1997. 15:71–9.
Article12). Akashi K., Mizuno S. Epstein-Barr virus-infected natural killer cell leukemia. Leuk Lymphoma. 2000. 40:57–66.
Article13). Kwong YL., Chan AC., Liang R, et al. CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol. 1997. 97:821–9.14). Takami A., Nakao S., Yachie A, et al. Successful treatment of Epstein-Barr virus-associated natural killer cell large granular lymphocytic leukaemia using allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998. 21:1279–82.
Article15). Teshima T., Miyaji R., Fukuda M., Ohshima K. Bone-marrow transplantation for Epstein-Barr-virus-associated natural killer cell-large granular lymphocyte leukaemia. Lancet. 1996. 347:1124.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epstein-Barr Virus-Associated Vesiculopapular Eruption on the Face of a Patient with Natural Killer T Cell Lymphoma
- Epstein Barr Virus-associated Vesicular Eruptions of the Face
- A Case of Hypersensitivity to Mosquito Bites without Peripheral Natural Killer Cell Lymphocytosis in a 6-Year-Old Korean Boy
- A Case of Hypersensitivity to Mosquito Bite Associated with Epstein-Barr Viral Infection and Natural Killer Cell Lymphocytosis
- Case of Chronic Active Epstein-Barr Virus Infection Developed Hemophagocytic Lymphohistiocytosis after COVID-19 Infection