Korean J Hematol.
2007 Jun;42(2):136-145. 10.5045/kjh.2007.42.2.136.
Anti-tumor Cytotoxicity of Allogeneic Neuroblastoma Tumor Antigen-loaded Dendiritic Cells
- Affiliations
-
- 1Pediatric Oncology Branch, Specific Organs Cancer Center, National Cancer Center, Korea.
- 2Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hsahn@snu.ac.kr
- KMID: 2083511
- DOI: http://doi.org/10.5045/kjh.2007.42.2.136
Abstract
-
BACKGROUND: Despite of aggressive treatments, the long-term survival rate is about 30% in stage 4 neuroblastoma (NBL). Recently, dendritic cell (DC)-based immunotherapy is emerging as a promising tool in cancer treatment. But it is very difficult to get sufficient amount of autologous tumor as the source of tumor antigen in DC-based immunotherapy. The purpose of this study was to test whether DCs loaded with allogeneic NBL tumor antigens can prime effective anti-tumor immune responses.
METHODS
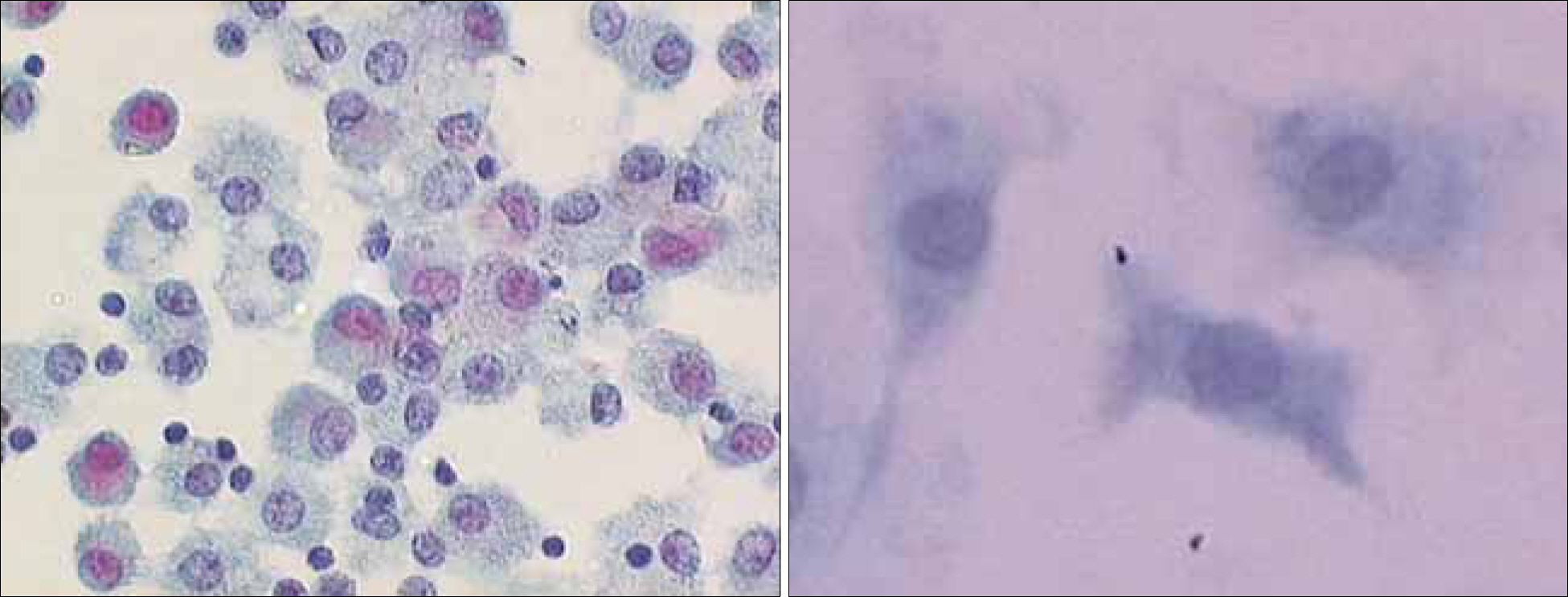
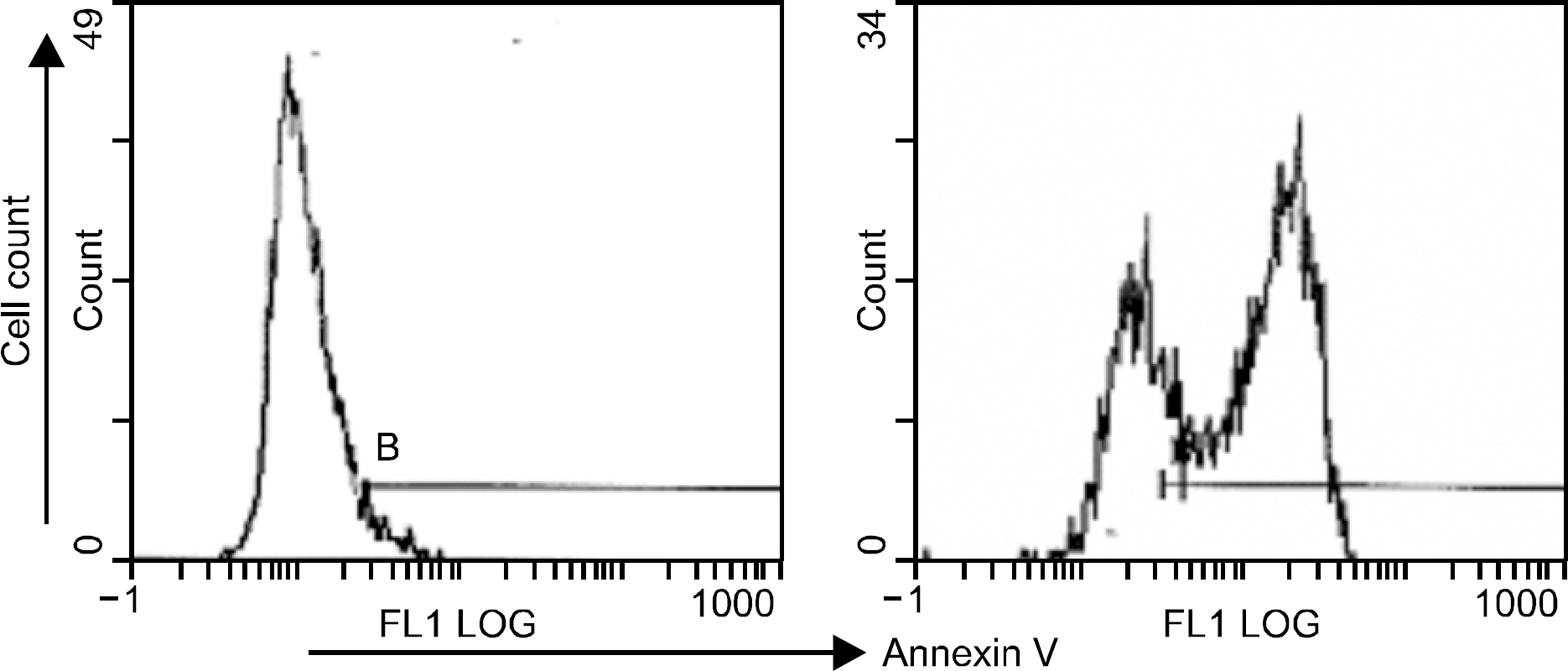
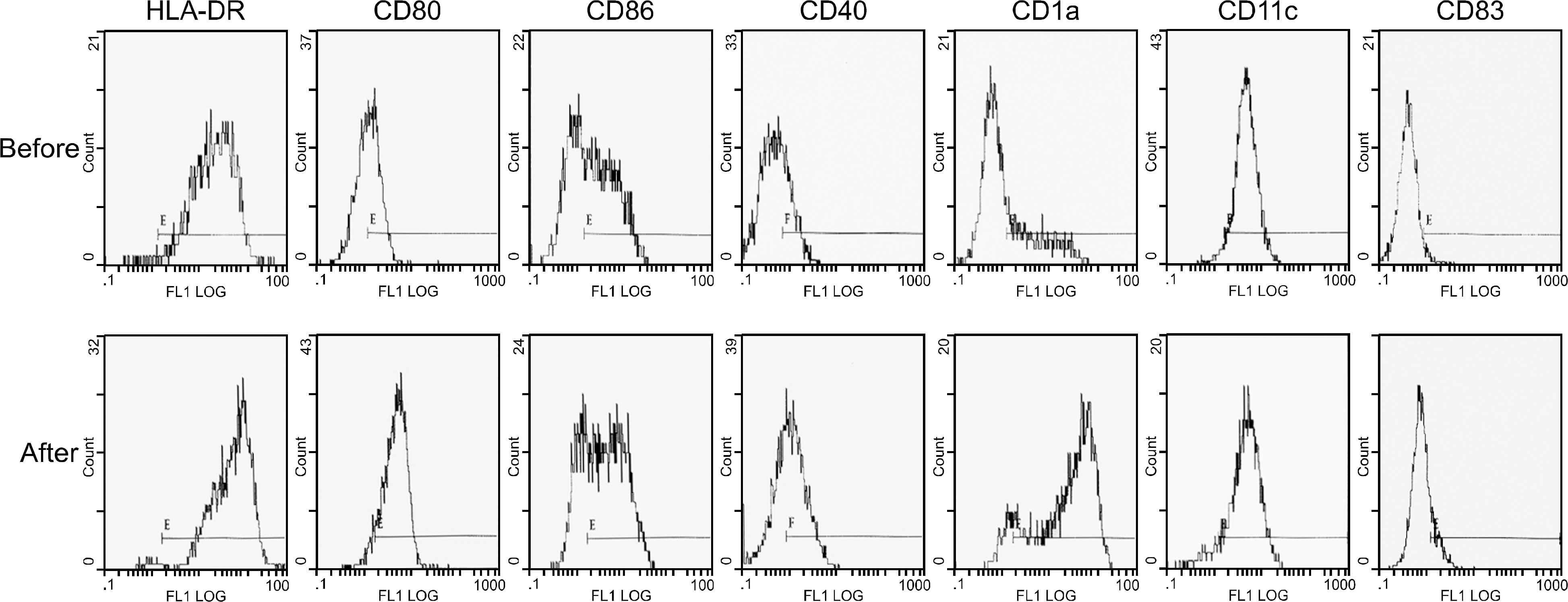
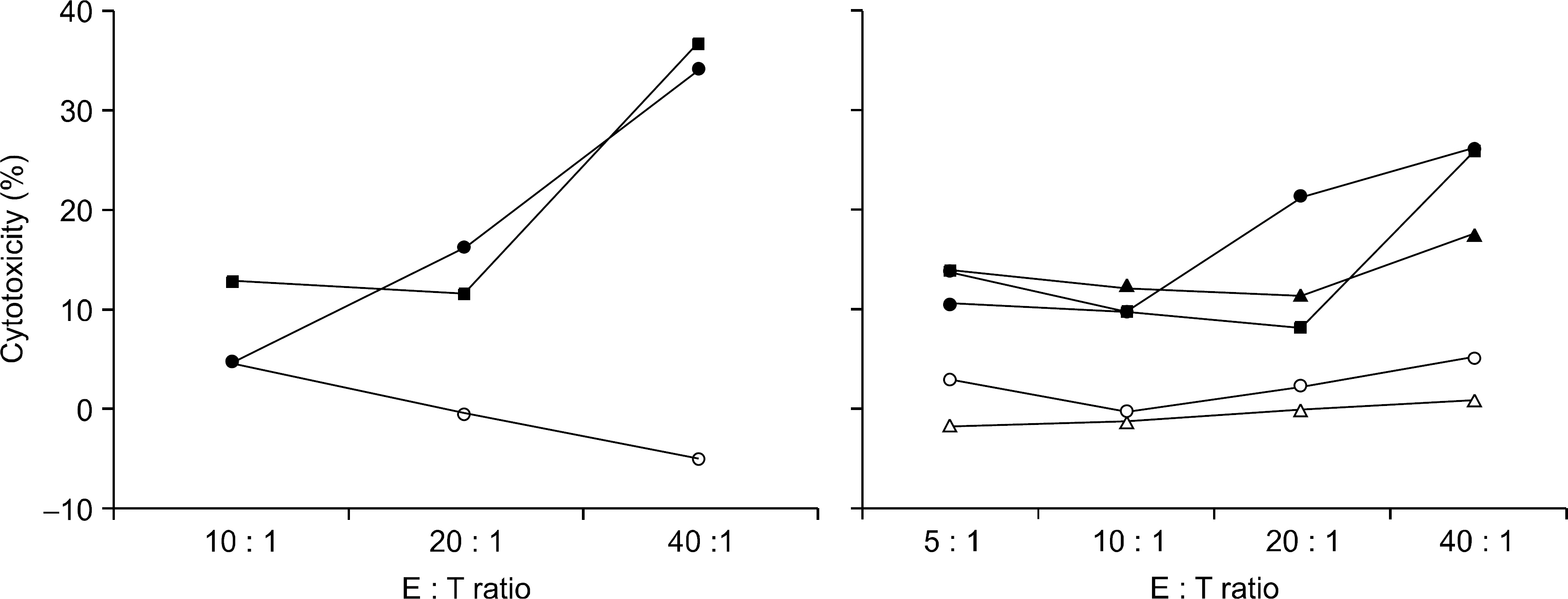
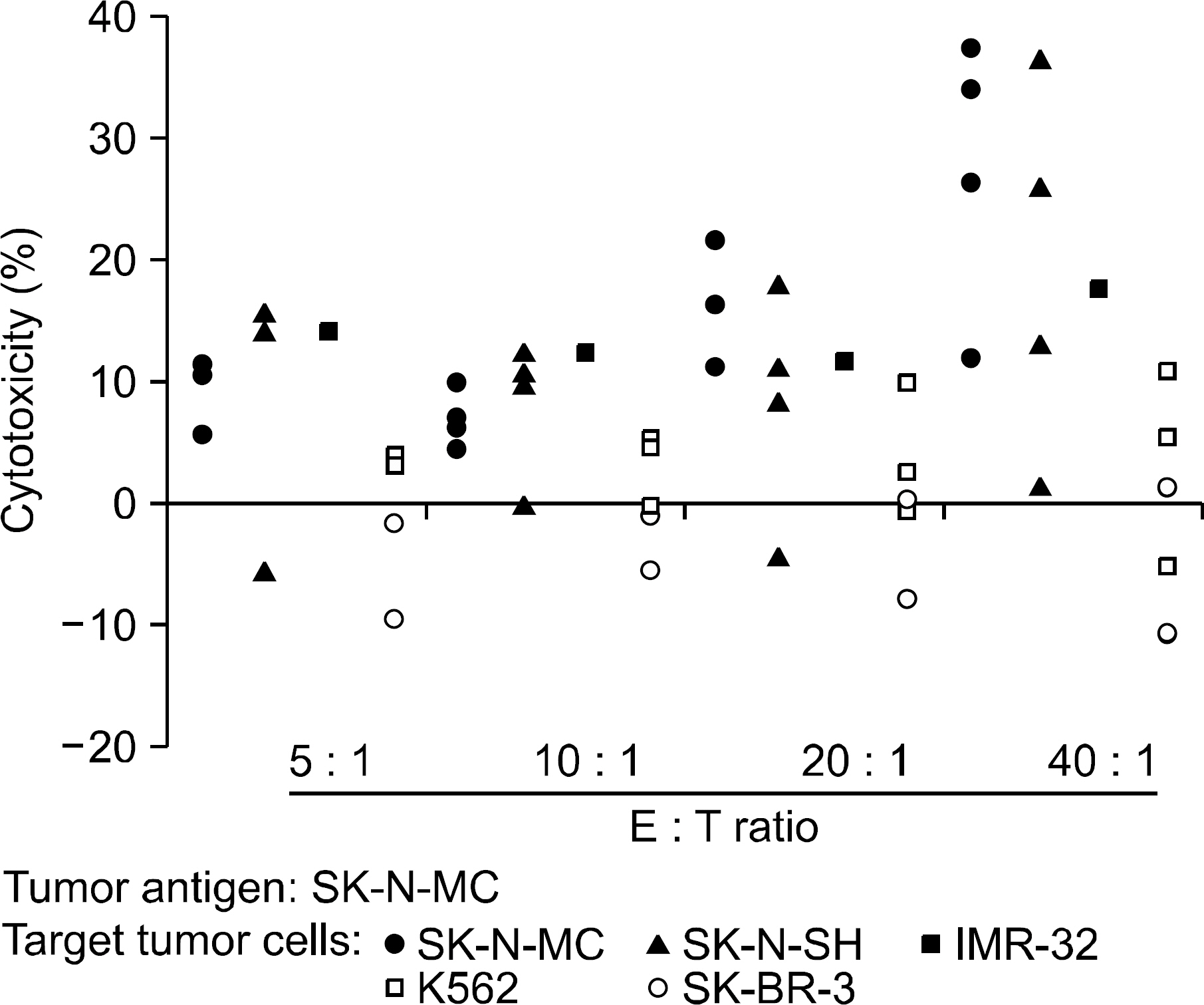
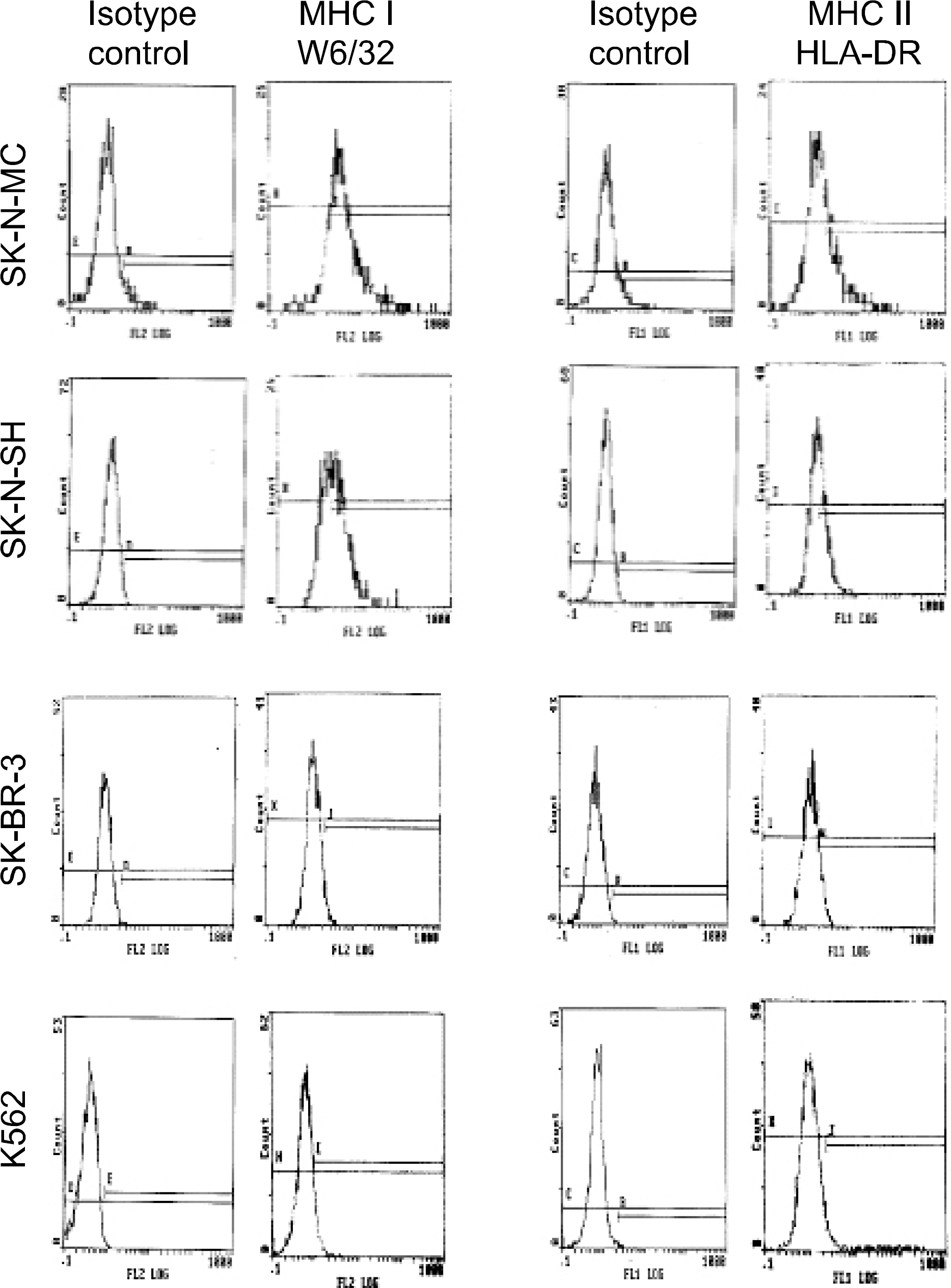
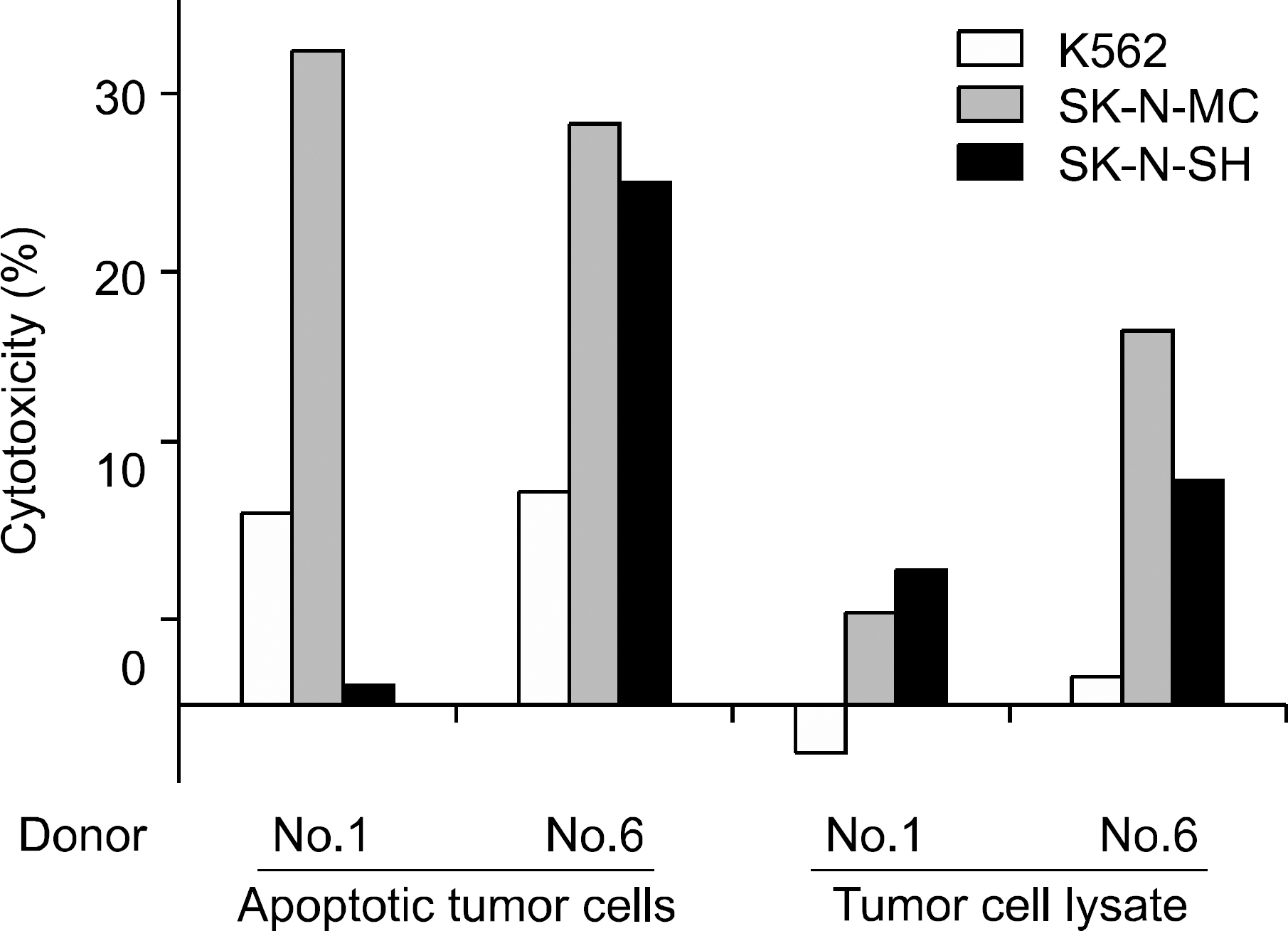
Peripheral blood mononuclear cells (PBMCs) were differentiated into immature DCs in the presence of GM-CSF and IL-4. As the source of tumor antigens, human neuroblastoma cell lines, SK-N-MC, SK-N-SH, and IMR-32, were used after induction of apoptosis by UV irradiation. The immature DCs were loaded with apoptotic tumor cells, and then cultured with PBMCs for priming the tumor antigen-specific T lymphocytes. The tumor-specific cytotoxicity of T lymphocytes against NBL cells was analysed after coculture.
RESULTS
Incubation of DCs with apoptotic tumor cells effectively loaded DCs with tumor antigens and induced maturation of DCs. The tumor antigen-challenged T lymphocytes effectively killed the NBL cells, which were used as tumor antigens. Furthermore, the T lymphocytes showed a broad ranged cytotoxicity to all of the NBL cell lines, which were not challenged as tumor antigens.
CONCLUSION
This study showed that the apoptotic NBL tumor cells induced maturation of DCs and could be used as tumor antigens, and DCs loaded with apoptotic NBL tumor cells could induce effective anti-tumor specific cytotoxic T lymphocytes to allogeneic NBL tumors.
MeSH Terms
Figure
Reference
-
1). Cheung NK., Kushner BH., LaQuaglia M, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001. 36:227–30.
Article2). Toren A., Nagler A., Rozenfeld-Granot G, et al. Amplification of immunological functions by subcutaneous injection of intermediate-high dose interleukin-2 for 2 years after autologous stem cell transplantation in children with stage IV neuroblastoma. Transplantation. 2000. 70:1100–4.
Article3). Abbas AK., Lichtman AH., Pober JS. Immunity to tumors. In: Cellular and molecular immunology. 3rd ed.Philadelphia: WB Saunders Co;1997. p. 382–405.4). Banchereau J., Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–52.
Article5). Brossart P., Wirths S., Brugger W., Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001. 29:1247–55.
Article6). Bowman LC., Grossmann M., Rill D, et al. Interleukin-2 gene-modified allogeneic tumor cells for treatment of relapsed neuroblastoma. Hum Gene Ther. 1998. 9:1303–11.
Article7). Berard F., Blanco P., Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2002. 192:1535–44.
Article8). Märten A., Greten T., Ziske C, et al. Generation of activated and antigen-specific T cells with cytotoxic activity after coculture with dendritic cells. Cancer Immunol Immunother. 2002. 51:25–32.9). Lee GK. Characterization of new monoclonal antibodies recognizing subsets of human dendritic cells. The 43rd Biannual Meeting of the Korean Association of Immunologists, Abstract (SIII-2), pp47-48. 2001.10). Kim JI., Lee HR., Kim EY., Kim SW., Lee GK., Song HG. Characterization of a new monoclonal antibody, CH6, against a human cell membrane molecule. The 42nd Biannual Meeting of the Korean Association of Immunologists, Abstract (P-06), p68. 2001.11). Thurner B., Haendle I., Röder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999. 190:1669–78.
Article12). Brossart P., Wirths S., Stuhler G., Reichardt VL., Kanz L., Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000. 96:3102–8.
Article13). Yu JS., Wheeler CJ., Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001. 61:842–7.14). Nabel GJ., Nabel EG., Yang ZY, et al. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993. 90:11307–11.
Article15). Nanda NK., Sercarz EE. Induction of anti-self-immunity to cure cancer. Cell. 1995. 82:13–7.
Article16). Pardoll D., Nabel GJ. Cancer immunotherapy. Friedmann T, editor. The development of human gene therapy. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1999. p. 427–57.17). Zhao XJ., Cheung NK. GD2 oligosaccharide: target for cytotoxic T lymphocytes. J Exp Med. 1995. 182:67–74.
Article18). Henderson RA., Finn OJ. Human tumor antigens are ready to fly. Adv Immunol. 1996. 62:217–56.
Article19). Foreman NK., Rill DR., Coustan-smith E., Douglass EC., Brenner MK. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br J Cancer. 1993. 67:933–8.
Article20). Cheung NK., Medof ME., Munn D. Immunotherapy with GD2 specific monoclonal antibodies. Prog Clin Biol Res. 1988. 271:619–32.21). Haight AE., Bowman LC., Ng CY., Vanin EF., Davi-doff AM. Humoral response to vaccination with interleukin-2-expressing allogeneic neuroblastoma cells after primary therapy. Med Pediatr Oncol. 2000. 35:712–5.
Article22). Eaton JD., Perry MJ., Nicholson S, et al. Allogeneic whole-cell vaccine: a phase I/II study in men with hormone-refractory prostate cancer. BJU Int. 2002. 89:19–26.
Article23). Melcher A., Todryk S., Bateman A., Chong H., Lemoi-ne NR., Vile RG. Adoptive transfer of immature dendritic cells with autologous or allogeneic tumor cells generates systemic antitumor immunity. Cancer Res. 1999. 59:2802–5.24). Shimizu K., Fields RC., Giedlin M., Mulé JJ. Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines. Proc Natl Acad Sci U S A. 1999. 96:2268–73.
Article25). Stift A., Friedl J., Dubsky P, et al. Dendritic cell-based vaccination in solid cancer. J Clin Oncol. 2003. 21:135–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-GD2 Monoclonal Antibody (dinutuximab) with GM-CSF, Interleukin 2, and Cis-retinoic Acid for the Treatment of High-risk Neuroblastoma
- Influence of primary tumor site on host anti-tumor immunity
- Tumor Vaccine Effect by IL-16 Gene Transfer into Murine Neuroblastoma Model
- The Effect of Mesenchymal Stem Cells on the Activation of Dendritic Cells in the Cell Culture Insert System
- The Effectiveness of IL-12 Administration and Fusion on Tumor Antigen Sensitization Methods for Dendritic Cells Derived from Patients with Myelogenous Leukemia