Korean J Obstet Gynecol.
2010 Jan;53(1):35-42. 10.5468/kjog.2010.53.1.35.
Comparison of cell growth suppression in SiHa cervical carcinoma cell line by human papillomavirus type 16 E6/E7 siRNAs
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ahnlab1@catholic.ac.kr
- 2Catholic Research Institutes of Medical Science, The Catholic University of Korea, Seoul, Korea.
- KMID: 2077958
- DOI: http://doi.org/10.5468/kjog.2010.53.1.35
Abstract
OBJECTIVE
Human cervical cancer is caused by the high-risk types of human papillomavirus (HPV) such as HPV16, which possess the E6 and E7 oncogenes, whose expressions are a prerequisite for cancer development. We performed this study to compare the efficacy of antitumor activity by HPV siRNA which silences only E6 or both E6/E7.
METHODS
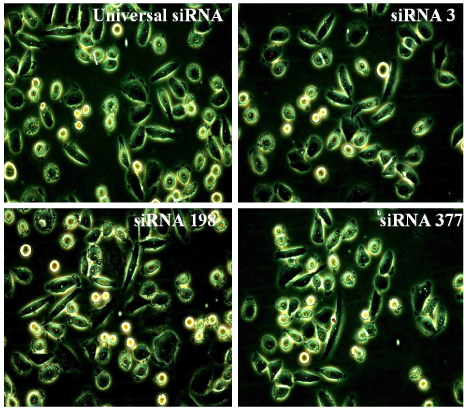
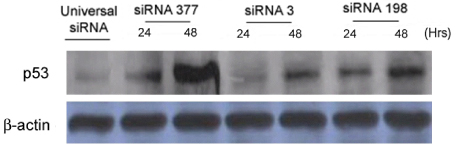
We transfected siRNA 377 (HPV16 E6 siRNA), siRNA 3 (HPV16 E6 siRNA), and siRNA 198 (HPV16 E7 siRNA) into SiHa cell line (siRNA 377 silences only E6, and siRNA 3 and siRNA 198 silence both E6 and E7). We experimented cell counts and morphologic changes 24 and 48 hours after transfection and expressions of HPV16 E6/E7 mRNA by RT-PCR.
RESULTS
siRNA 377, siRNA 3, and siRNA 198 suppressed the cell growth. siRNA 3 and siRNA 198 were more potent than siRNA 377 in cell growth suppression. siRNA 377 knocked down the expression of E6 mRNA, and both siRNA 3 and siRNA 198 knocked down the expression of E6/E7 mRNA.
CONCLUSION
Our results suggest that simultaneous suppression of E6 and E7 was more potent than E6-specific suppression in cancer cell growth.
Keyword
MeSH Terms
Figure
Reference
-
1. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002. 2:342–350.2. Fen J, Yoshinouchi M, Nakamura K, Kodama J, Nasu Y, Yamato K, et al. Eradication of HPV post-surgical treatments, its correlation with specific types, types of surgery and the physical status. Oncol Rep. 2004. 12:375–379.3. Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995. 87:796–802.4. zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994. 186:131–156.5. Huibregtse JM, Scheffner M, Howley PM. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993. 13:4918–4927.6. Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993. 13:775–784.7. Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989. 8:4099–4105.8. Dyson N, Howley PM, Münger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989. 243:934–937.9. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001. 411:494–498.10. Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001. 114(Pt 24):4557–4565.11. Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002. 21:6041–6048.12. Yamato K, Fen J, Kobuchi H, Nasu Y, Yamada T, Nishihara T, et al. Induction of cell death in human papillomavirus 18-positive cervical cancer cells by E6 siRNA. Cancer Gene Ther. 2006. 13:234–241.13. Yoshinouchi M, Yamada T, Kizaki M, Fen J, Koseki T, Ikeda Y, et al. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol Ther. 2003. 8:762–768.14. Tang S, Tao M, McCoy JP Jr, Zheng ZM. Short-term induction and long-term suppression of HPV16 oncogene silencing by RNA interference in cervical cancer cells. Oncogene. 2006. 25:2094–2104.15. Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene. 2003. 22:5938–5945.16. de Villiers EM. Heterogeneity of the human papillomavirus group. J Virol. 1989. 63:4898–4903.17. von Knebel Doeberitz M, Oltersdorf T, Schwarz E, Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988. 48:3780–3786.18. Scheffner M, Whitaker NJ. Human papillomavirus-induced carcinogenesis and the ubiquitin-proteasome system. Semin Cancer Biol. 2003. 13:59–67.19. Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000. 267:141–150.20. Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991. 6:1915–1922.21. Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999. 80(Pt 6):1513–1517.22. Gross-Mesilaty S, Reinstein E, Bercovich B, Tobias KE, Schwartz AL, Kahana C, et al. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc Natl Acad Sci U S A. 1998. 95:8058–8063.23. Steele C, Cowsert LM, Shillitoe EJ. Effects of human papillomavirus type 18-specific antisense oligonucleotides on the transformed phenotype of human carcinoma cell lines. Cancer Res. 1993. 53:10 Suppl. 2330–2337.24. Venturini F, Braspenning J, Homann M, Gissmann L, Sczakiel G. Kinetic selection of HPV 16 E6/E7-directed antisense nucleic acids: anti-proliferative effects on HPV 16-transformed cells. Nucleic Acids Res. 1999. 27:1585–1592.25. Alvarez-Salas LM, Cullinan AE, Siwkowski A, Hampel A, DiPaolo JA. Inhibition of HPV-16 E6/E7 immortalization of normal keratinocytes by hairpin ribozymes. Proc Natl Acad Sci U S A. 1998. 95:1189–1194.26. Chen Z, Kamath P, Zhang S, St John L, Adler-Storthz K, Shillitoe EJ. Effects on tumor cells of ribozymes that cleave the RNA transcripts of human papillomavirus type 18. Cancer Gene Ther. 1996. 3:18–23.27. Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985. 314:111–114.28. Sima N, Wang W, Kong D, Deng D, Xu Q, Zhou J, et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of Rb and p53. Apoptosis. 2008. 13:273–281.29. Kösel S, Burggraf S, Engelhardt W, Olgemöller B. Increased levels of HPV16 E6*I transcripts in high-grade cervical cytology and histology (CIN II+) detected by rapid real-time RT-PCR amplification. Cytopathology. 2007. 18:290–299.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and localization of human papillomavirus type 16 E6 and E7 open reading frame proteins in human epidermal keratinocyte
- Human Papillomavirus Infection
- Clinical Significance of Expression of p53 Oncoprotein and Human Papillomavirus in Cervical Cancer
- Long (27-nucleotides) small inhibitory RNAs targeting E6 protein eradicate effectively the cervical cancer cells harboring human papilloma virus
- The effects of wild type p53 tumor suppressor gene expression on the normal human cervical epithelial cells or human epidermal keratinocytes transformed with human papillomavirus type 16 DNA