Korean J Physiol Pharmacol.
2012 Apr;16(2):119-123. 10.4196/kjpp.2012.16.2.119.
Effect of Agrimonia pilosa Ledeb Extract on the Antinociception and Mechanisms in Mouse
- Affiliations
-
- 1Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- 2Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- 3Department of Pharmacology, College of Medicine, Translational Research Center, Institute of Bio-Science and Technology, Dankook University, Cheonan 330-714, Korea.
- KMID: 2071757
- DOI: http://doi.org/10.4196/kjpp.2012.16.2.119
Abstract
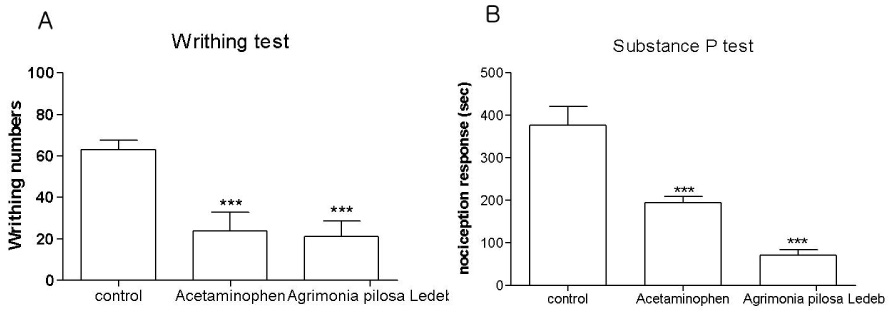
- In the present study, the antinociceptive profiles of Agrimonia pilosa Ledeb extract were examined in ICR mice. Agrimonia pilosa Ledeb extract administered orally (200 mg/kg) showed an antinociceptive effect as measured by the tail-flick and hot-plate tests. In addition, Agrimonia pilosa Ledeb extract attenuated the writhing numbers in the acetic acid-induced writhing test. Furthermore, the cumulative nociceptive response time for intrathecal (i.t.) injection of substance P (0.7 microg) was diminished by Agrimonia pilosa Ledeb extract. Intraperitoneal (i.p.) pretreatment with yohimbine (alpha2-adrenergic receptor antagonist) attenuated antinociceptive effect induced by Agrimonia pilosa Ledeb extract in the writhing test. However, naloxone (opioid receptor antagonist) or methysergide (5-HT serotonergic receptor antagonist) did not affect antinociception induced by Agrimonia pilosa Ledeb extract in the writhing test. Our results suggest that Agrimonia pilosa Ledeb extract shows an antinociceptive property in various pain models. Furthermore, this antinociceptive effect of Agrimonia pilosa Ledeb extract may be mediated by alpha2-adrenergic receptor, but not opioidergic and serotonergic receptors.
MeSH Terms
Figure
Reference
-
1. Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK. Anti-inflammatory bioactivities in plant extracts. J Med Food. 2007. 10:1–10.2. Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010. 30:411–424.3. Kato H, Li W, Koike M, Wang Y, Koike K. Phenolic glycosides from Agrimonia pilosa. Phytochemistry. 2010. 71:1925–1929.4. Koshiura R, Miyamoto K, Ikeya Y, Taguchi H. Antitumor activity of methanol extract from roots of Agrimonia pilosa Ledeb. Jpn J Pharmacol. 1985. 38:9–16.5. Miyamoto K, Kishi N, Koshiura R. Antitumor effect of agrimoniin, a tannin of Agrimonia pilosa Ledeb., on transplantable rodent tumors. Jpn J Pharmacol. 1987. 43:187–195.6. Li Y, Ooi LS, Wang H, But PP, Ooi VE. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother Res. 2004. 18:718–722.7. Shin WJ, Lee KH, Park MH, Seong BL. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol. 2010. 54:11–19.8. Zhu L, Tan J, Wang B, He R, Liu Y, Zheng C. Antioxidant activities of aqueous extract from Agrimonia pilosa Ledeb and its fractions. Chem Biodivers. 2009. 6:1716–1726.9. Yamaki M, Kashihara M, Ishiguro K, Takagi S. Antimicrobial Principles of Xian he cao (Agrimonia pilosa). Planta Med. 1989. 55:169–170.10. Jung CH, Zhou S, Ding GX, Kim JH, Hong MH, Shin YC, Kim GJ, Ko SG. Antihyperglycemic activity of herb extracts on streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2006. 70:2556–2559.11. Jung CH, Kim JH, Park S, Kweon DH, Kim SH, Ko SG. Inhibitory effect of Agrimonia pilosa Ledeb. on inflammation by suppression of iNOS and ROS production. Immunol Invest. 2010. 39:159–170.12. Bae H, Kim HJ, Shin M, Lee H, Yin CS, Ra J, Kim J. Inhibitory effect of Agrimoniae Herba on lipopolysaccharide-induced nitric oxide and proinflammatory cytokine production in BV2 microglial cells. Neurol Res. 2010. 32:Suppl 1. 53–57.13. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980. 67:313–316.14. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981. 217:212–215.15. D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941. 72:74–79.16. Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953. 107:385–393.17. Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Federal Proceeding. 1959. 18:412.18. Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003. 73:471–485.19. Park SH, Sim YB, Choi SM, Seo YJ, Kwon MS, Lee JK, Suh HW. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch Pharm Res. 2009. 32:1643–1649.20. Suh HW, Song DK, Son KH, Wie MB, Lee KH, Jung KY, Do JC, Kim YH. Antinociceptive mechanisms of dipsacus saponin C administered intracerebroventricularly in the mouse. Gen Pharmacol. 1996. 27:1167–1172.21. Suh HW, Song DK, Kim YH. Differential effects of adenosine receptor antagonists injected intrathecally on antinociception induced by morphine and beta-endorphin administered intracerebroventricularly in the mouse. Neuropeptides. 1997. 31:339–344.22. Suh HW, Chung KM, Kim YH, Huh SO, Song DK. Effects of histamine receptor antagonists injected intrathecally on antinociception induced by opioids administered intracerebroventricularly in the mouse. Neuropeptides. 1999. 33:121–129.23. Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985. 22:1–31.24. Grumbach L. Knighton RS, Dumke PR, editors. The prediction of analgesic activity in man by animal testing. Pain. 1966. Boston: Little Brown and Co.;163–182.25. Vyklicky L. Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. The techniques for the study of pain in animals. Advances in Pain Research and Theraphy. 1979. Vol. 3. New York: Raven Press;727–745.26. Cumberbatch MJ, Herrero JF, Headley PM. Exposure of rat spinal neurones to NMDA, AMPA and kainate produces only short-term enhancements of responses to noxious and non-noxious stimuli. Neurosci Lett. 1994. 181:98–102.27. Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984. 228:1–12.28. Yaksh TL. Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinociceptive effects of morphine in the periaqueductal gray. Brain Res. 1979. 160:180–185.29. Yaksh TL. Multiple opioid receptor systems in brain and spinal cord: Part I. Eur J Anaesthesiol. 1984. 1:171–199.30. Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984. 321:287–297.31. Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987. 242:90–95.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Agrimonia Pilosa Ledeb. Extract on the Growth of Food-Borne Pathogens
- Chemical Constituents from Agrimonia pilosa with Inhibitory Activity against Interleukin 1β Production via NLRP3 and NLRC4 Inflammasomes
- Antinociception Effect and Mechanisms of Campanula Punctata Extract in the Mouse
- Hop Extract Produces Antinociception by Acting on Opioid System in Mice
- The Molecular Signatures of Acute-immobilization-induced Antinociception and Chronic-immobilization-induced Antinociceptive Tolerance