Korean J Physiol Pharmacol.
2015 Nov;19(6):549-555. 10.4196/kjpp.2015.19.6.549.
Multiple Signaling Pathways Contribute to the Thrombin-induced Secretory Phenotype in Vascular Smooth Muscle Cells
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Yangsan 50612, Korea. koanhoi@pusan.ac.kr
- 2College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Iksan 54596, Korea.
- KMID: 2070795
- DOI: http://doi.org/10.4196/kjpp.2015.19.6.549
Abstract
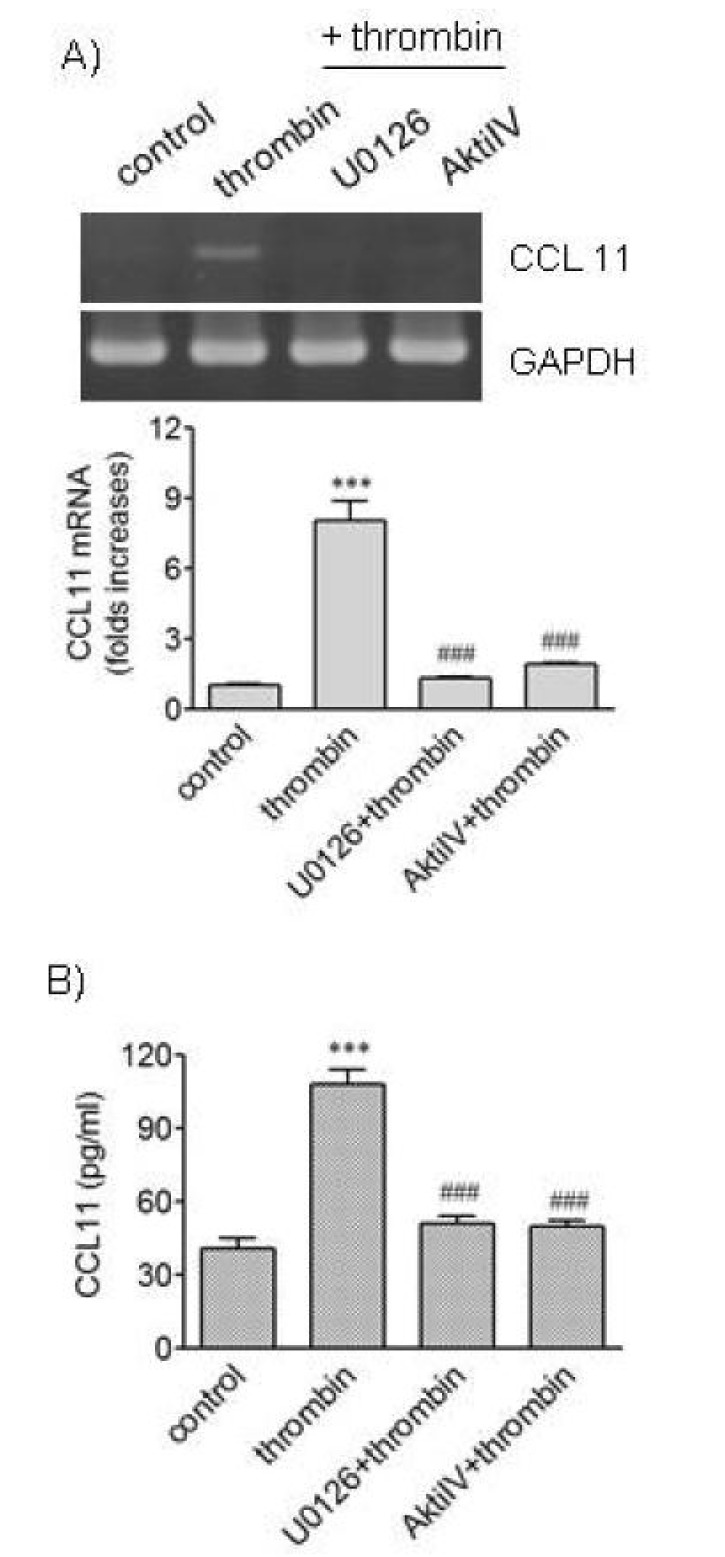
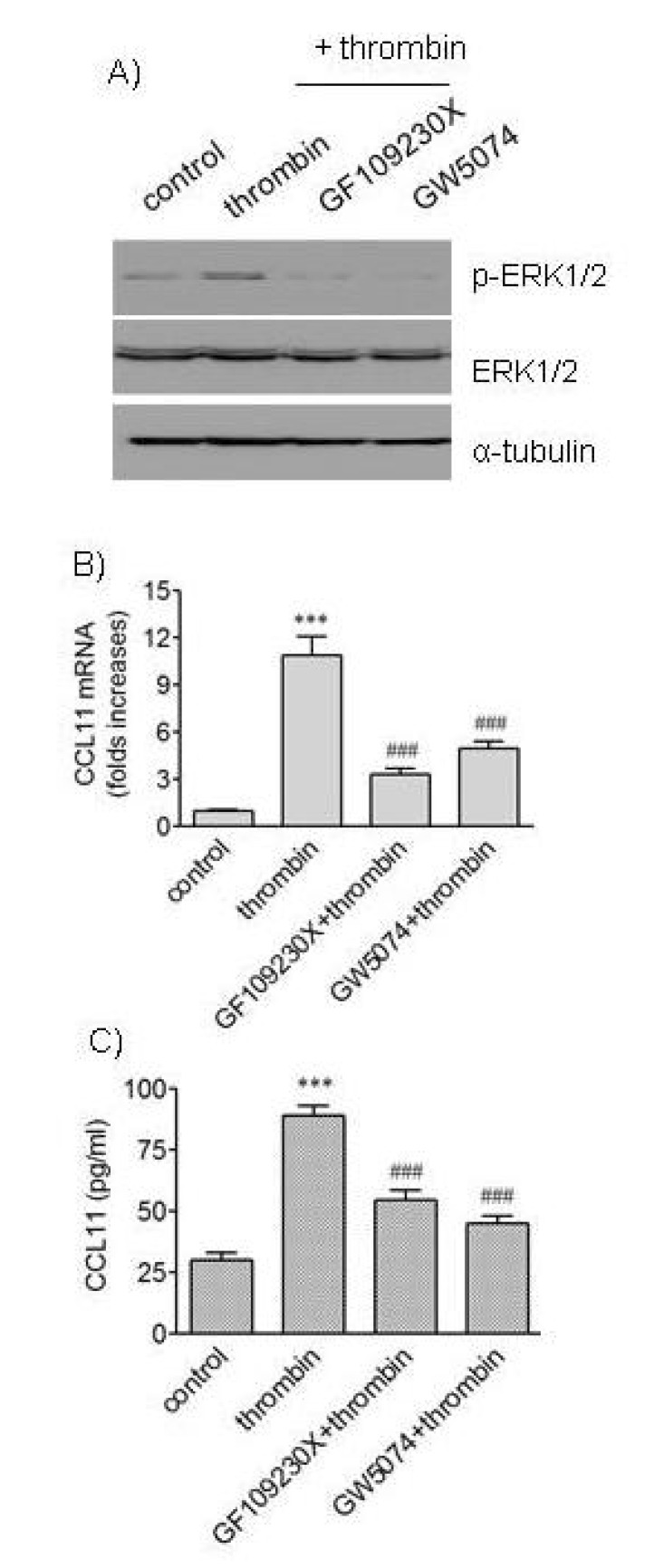
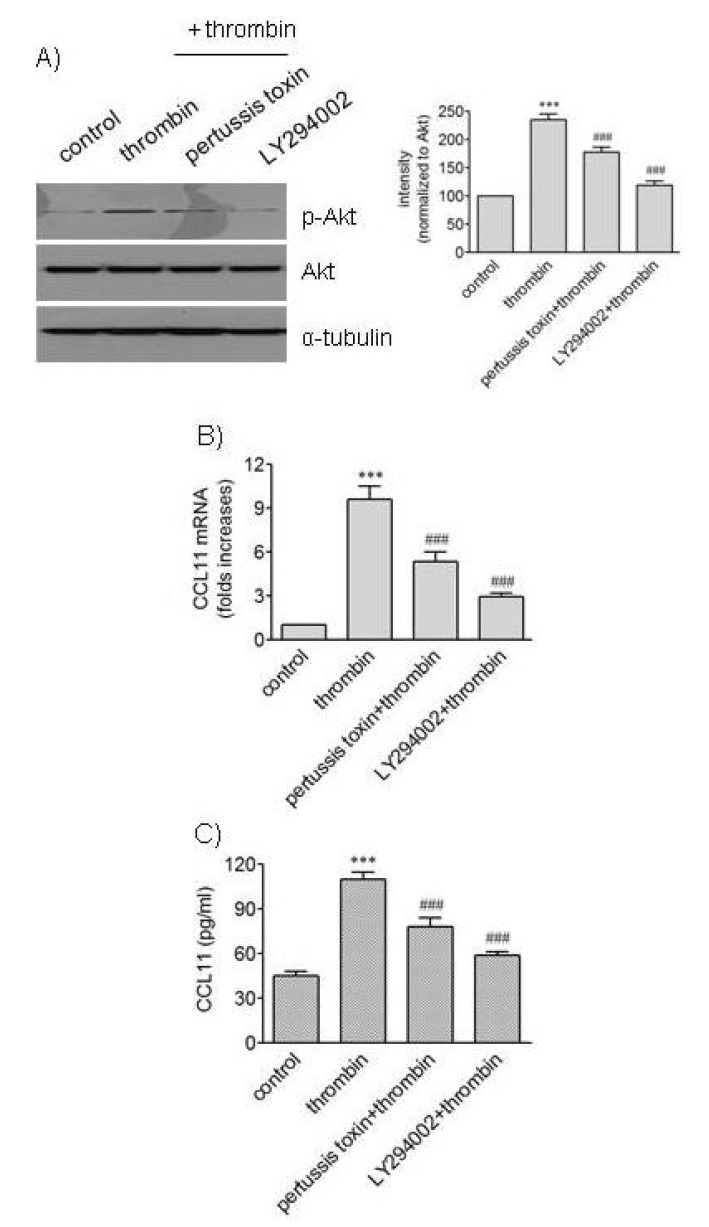
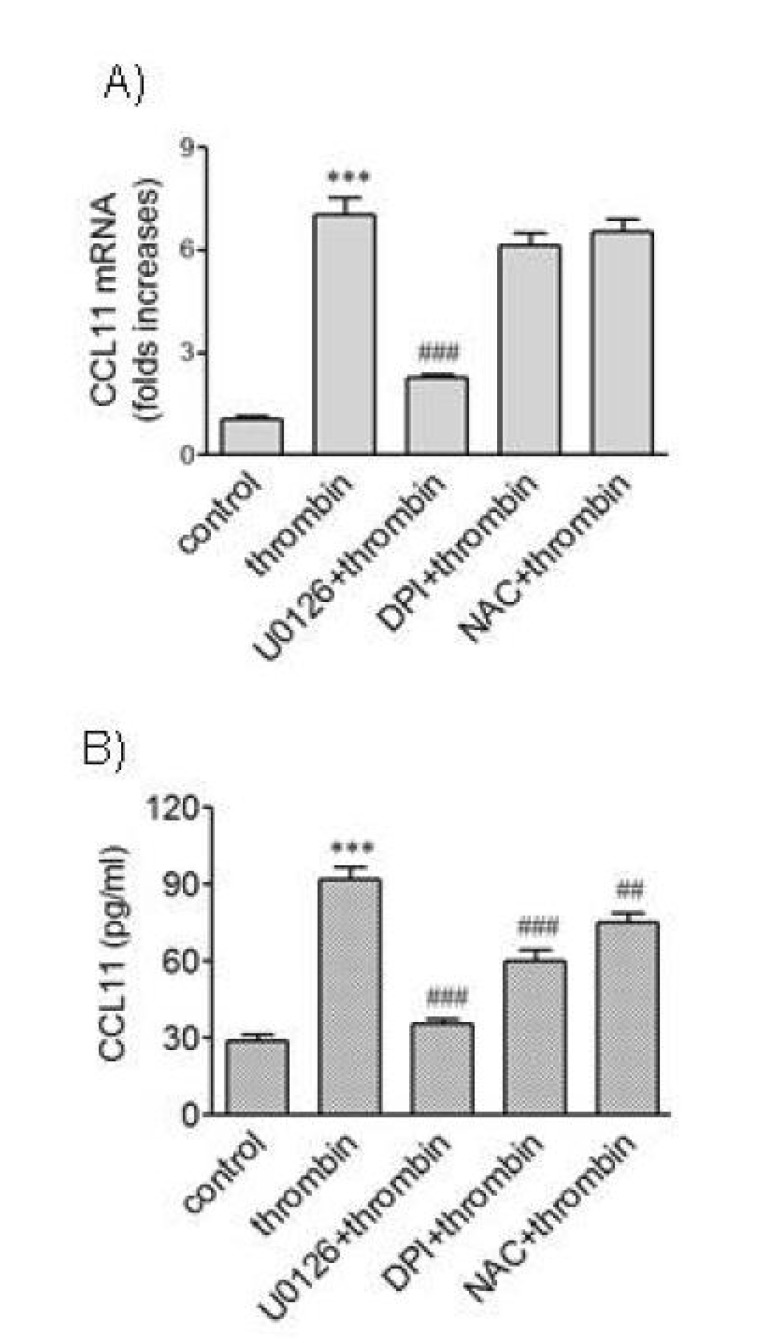
- We attempted to investigate molecular mechanisms underlying phenotypic change of vascular smooth muscle cells (VSMCs) by determining signaling molecules involved in chemokine production. Treatment of human aortic smooth muscle cells (HAoSMCs) with thrombin resulted not only in elevated transcription of the (C-C motif) ligand 11 (CCL11) gene but also in enhanced secretion of CCL11 protein. Co-treatment of HAoSMCs with GF109230X, an inhibitor of protein kinase C, or GW5074, an inhibitor of Raf-1 kinase, caused inhibition of ERK1/2 phosphorylation and significantly attenuated expression of CCL11 at transcriptional and protein levels induced by thrombin. Both Akt phosphorylation and CCL11 expression induced by thrombin were attenuated in the presence of pertussis toxin (PTX), an inhibitor of Gi protein-coupled receptor, or LY294002, a PI3K inhibitor. In addition, thrombin-induced production of CCL11 was significantly attenuated by pharmacological inhibition of Akt or MEK which phosphorylates ERK1/2. These results indicate that thrombin is likely to promote expression of CCL11 via PKC/Raf-1/ERK1/2 and PTX-sensitive protease-activated receptors/PI3K/Akt pathways in HAoSMCs. We propose that multiple signaling pathways are involved in change of VSMCs to a secretory phenotype.
MeSH Terms
Figure
Reference
-
1. Jakubowski HV, Owen WG. Macromolecular specificity determinants on thrombin for fibrinogen and thrombomodulin. J Biol Chem. 1989; 264:11117–11121. PMID: 2544585.
Article2. Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000; 407:258–264. PMID: 11001069.
Article3. Chambers RC, Laurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans. 2002; 30:194–200. PMID: 12023850.
Article4. Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001; 53:245–282. PMID: 11356985.5. Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991; 64:1057–1068. PMID: 1672265.
Article6. Bretschneider E, Kaufmann R, Braun M, Nowak G, Glusa E, Schrör K. Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol. 2001; 132:1441–1446. PMID: 11264237.
Article7. Bretschneider E, Kaufmann R, Braun M, Wittpoth M, Glusa E, Nowak G, Schrör K. Evidence for proteinase-activated receptor-2 (PAR-2)-mediated mitogenesis in coronary artery smooth muscle cells. Br J Pharmacol. 1999; 126:1735–1740. PMID: 10372815.
Article8. Chung SW, Park JW, Lee SA, Eo SK, Kim K. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun. 2010; 396:748–754. PMID: 20451499.
Article9. Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008; 28:1909–1919. PMID: 18669888.
Article10. Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007; 97:730–737. PMID: 17479183.
Article11. Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999; 10:61–86. PMID: 10379912.
Article12. Chen J, Akyürek LM, Fellström B, Häyry P, Paul LC. Eotaxin and capping protein in experimental vasculopathy. Am J Pathol. 1998; 153:81–90. PMID: 9665468.
Article13. Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, Thompson JF, Sukhova GH, Libby P, Lee RT. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000; 102:2185–2189. PMID: 11056090.14. Nelken NA, Soifer SJ, O'Keefe J, Vu TK, Charo IF, Coughlin SR. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992; 90:1614–1621. PMID: 1328304.
Article15. Schrör K, Bretschneider E, Fischer K, Fischer JW, Pape R, Rauch BH, Rosenkranz AC, Weber AA. Thrombin receptors in vascular smooth muscle cells - function and regulation by vasodilatory prostaglandins. Thromb Haemost. 2010; 103:884–890. PMID: 20143010.
Article16. Bar-Shavit R, Eldor A, Vlodavsky I. Binding of thrombin to subendothelial extracellular matrix. Protection and expression of functional properties. J Clin Invest. 1989; 84:1096–1104. PMID: 2794047.
Article17. Ivey ME, Little PJ. Thrombin regulates vascular smooth muscle cell proteoglycan synthesis via PAR-1 and multiple downstream signalling pathways. Thromb Res. 2008; 123:288–297. PMID: 18571697.
Article18. Marutsuka K, Hatakeyama K, Sato Y, Yamashita A, Sumiyoshi A, Asada Y. Protease-activated receptor 2 (PAR2) mediates vascular smooth muscle cell migration induced by tissue factor/factor VIIa complex. Thromb Res. 2002; 107:271–276. PMID: 12479889.
Article19. Ueda Y, Hirai Si, Osada Si, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996; 271:23512–23519. PMID: 8798560.20. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004; 30:193–204. PMID: 15023437.21. Kanthou C, Kanse SM, Kakkar VV, Benzakour O. Involvement of pertussis toxin-sensitive and -insensitive G proteins in alpha-thrombin signalling on cultured human vascular smooth muscle cells. Cell Signal. 1996; 8:59–66. PMID: 8777142.22. Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004; 36:49–56. PMID: 14734047.
Article23. Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001; 166:7571–7578. PMID: 11390513.24. Kodali RB, Kim WJ, Galaria II, Miller C, Schecter AD, Lira SA, Taubman MB. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2004; 24:1211–1216. PMID: 15130922.
Article25. Bretschneider E, Spanbroek R, Lötzer K, Habenicht AJ, Schrör K. Evidence for functionally active protease-activated receptor-3 (PAR-3) in human vascular smooth muscle cells. Thromb Haemost. 2003; 90:704–709. PMID: 14515192.
Article26. Bassus S, Herkert O, Kronemann N, Görlach A, Bremerich D, Kirchmaier CM, Busse R, Schini-Kerth VB. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: role of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2001; 21:1550–1555. PMID: 11557687.27. Wu SQ, Aird WC. Thrombin, TNF-alpha, and LPS exert overlapping but nonidentical effects on gene expression in endothelial cells and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005; 289:H873–H885. PMID: 15833800.28. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994; 91:9208–9212. PMID: 7937743.
Article29. Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005; 67:2–11. PMID: 15371558.
Article30. Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003; 17:1263–1293. PMID: 12835716.
Article31. McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007; 1773:1263–1284. PMID: 17126425.
Article32. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003; 42:1075–1081. PMID: 14581295.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Contribution of Resident Vascular Stem Cells to Arterial Pathology
- HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- Mechanism of Developing Diabetic Vascular Complication by Oxidative Stress
- Thrombin-induced Migration and Matrix Metalloproteinase-9 Expression Are Regulated by MAPK and PI3K Pathways in C6 Glioma Cells
- The role of sex steroid hormones in the pathophysiology and treatment of sarcopenia