Yonsei Med J.
2014 Nov;55(6):1631-1639. 10.3349/ymj.2014.55.6.1631.
Epidural Dexamethasone Decreased Inflammatory Hyperalgesia and Spinal cPLA2 Expression in a Rat Formalin Test
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea University College of Medicine, Seoul, Korea. anejhkim@korea.ac.kr
- KMID: 2070213
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1631
Abstract
- PURPOSE
The aim of this study was to investigate the effect of epidural dexamethasone on analgesia and cytosolic phospholipase A2 (cPLA2) expression in the spinal cord in a rat formalin test.
MATERIALS AND METHODS
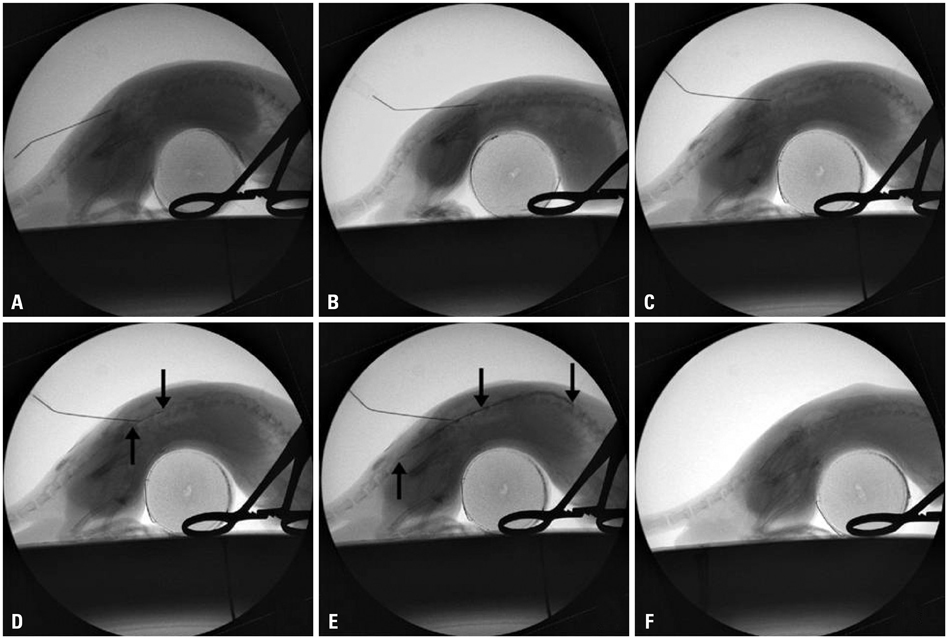
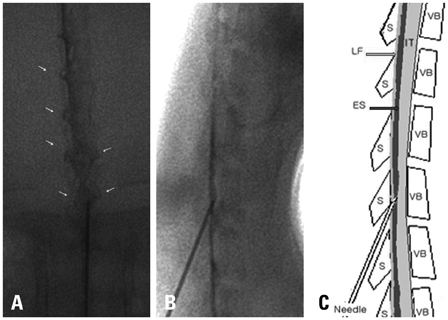
Epidural dexamethasone injection was performed to Sprague-Dawley rats with a 25 gauge needle under fluoroscopy. Following the epidural injection, a formalin induced pain behavior test was performed. Next, the spinal cords corresponding to L4 dorsal root ganglion was extracted to observe the cPLA2 expression.
RESULTS
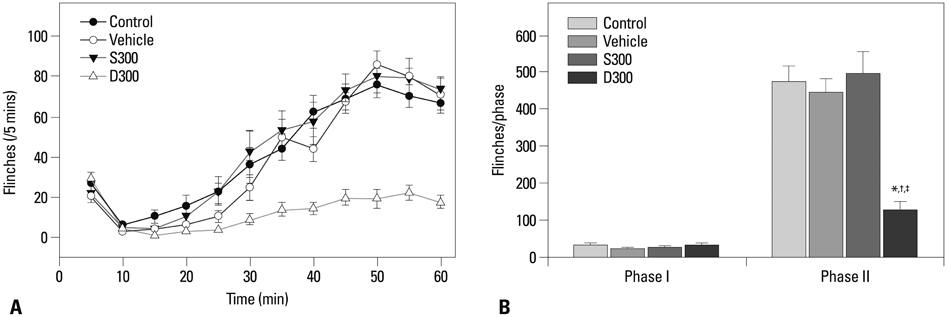
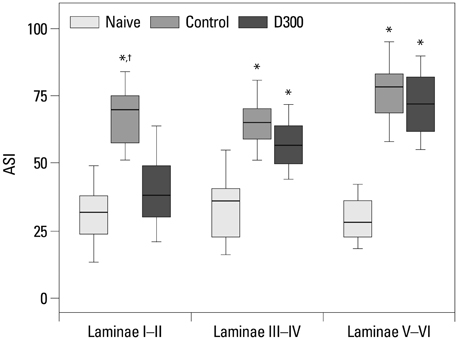
There were no differences in pain response during phase I among the groups. The phase II pain response in 300 microg of epidural dexamethasone group decreased as compared to control, 30 microg of epidural dexamethasone, 100 microg of epidural dexamethasone, and 300 microg of systemic dexamethasone groups. The expression of cPLA2 decreased in Rexed laminae I-II in 300 microg of the epidural dexamethasone group compared with the ones in the control group.
CONCLUSION
Taken together, these results suggest that 300 microg of epidural dexamethasone has an attenuating effect on the peripheral inflammatory tissue injury induced hyperalgesia and this effect is mediated through the inhibition of intraspinal cPLA2 expression and the primary site of action is the laminae I-II of the spinal cord.
MeSH Terms
-
Animals
Anti-Inflammatory Agents/*pharmacology
Dexamethasone/*pharmacology
Formaldehyde/*adverse effects
Group IV Phospholipases A2/*metabolism
Hyperalgesia/*drug therapy
Injections, Epidural
Male
Pain/chemically induced/*metabolism
Pain Measurement
Rats
Rats, Sprague-Dawley
Spinal Cord/*metabolism
Anti-Inflammatory Agents
Dexamethasone
Formaldehyde
Group IV Phospholipases A2
Figure
Reference
-
1. Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003; 63:855–867.
Article2. Qi HY, Daniels MP, Liu Y, Chen LY, Alsaaty S, Levine SJ, et al. A cytosolic phospholipase A2-initiated lipid mediator pathway induces autophagy in macrophages. J Immunol. 2011; 187:5286–5292.
Article3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–1723.
Article4. De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003; 24:488–522.
Article5. Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007; 10:185–212.6. Sluka KA, Dougherty PM, Sorkin LS, Willis WD, Westlund KN. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res Brain Res Rev. 1992; 17:29–38.
Article7. Kidd BL, Morris VH, Urban L. Pathophysiology of joint pain. Ann Rheum Dis. 1996; 55:276–283.
Article8. Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001; 276:30150–30160.
Article9. Kim DH, Fitzsimmons B, Hefferan MP, Svensson CI, Wancewicz E, Monia BP, et al. Inhibition of spinal cytosolic phospholipase A(2) expression by an antisense oligonucleotide attenuates tissue injury-induced hyperalgesia. Neuroscience. 2008; 154:1077–1087.
Article10. Ayoub SS, Yazid S, Flower RJ. Increased susceptibility of annexin-A1 null mice to nociceptive pain is indicative of a spinal antinociceptive action of annexin-A1. Br J Pharmacol. 2008; 154:1135–1142.
Article11. Thomas S, Beevi S. Epidural dexamethasone reduces postoperative pain and analgesic requirements. Can J Anaesth. 2006; 53:899–905.
Article12. Khafagy HF, Refaat AI, El-Sabae HH, Youssif MA. Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia. J Anesth. 2010; 24:531–536.
Article13. Jo YY, Yoo JH, Kim HJ, Kil HK. The effect of epidural administration of dexamethasone on postoperative pain: a randomized controlled study in radical subtotal gastrectomy. Korean J Anesthesiol. 2011; 61:233–237.
Article14. Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994; 81:1198–1205.
Article15. Yoon MH, Park KD, Lee HG, Kim WM, An TH, Kim YO, et al. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J Korean Med Sci. 2008; 23:1033–1038.
Article16. Kim MJ, Lee WH, Ko YK, Hong BH. Antinociceptive drug interaction between intrathecal vitamin E and gabapentin in the rat formalin test. Korean J Anesthesiol. 2012; 63:447–453.
Article17. Ong WY, Horrocks LA, Farooqui AA. Immunocytochemical localization of cPLA2 in rat and monkey spinal cord. J Mol Neurosci. 1999; 12:123–130.
Article18. Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, et al. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol. 2007; 31:401–407.
Article19. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51:5–17.
Article20. Bernards CM, Hill HF. Physical and chemical properties of drug molecules governing their diffusion through the spinal meninges. Anesthesiology. 1992; 77:750–756.
Article21. Bernards CM, Sorkin LS. Radicular artery blood flow does not redistribute fentanyl from the epidural space to the spinal cord. Anesthesiology. 1994; 80:872–878.
Article22. Hayashi N, Weinstein JN, Meller ST, Lee HM, Spratt KF, Gebhart GF. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976). 1998; 23:877–885.
Article23. Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth. 2001; 48:1000–1010.
Article24. Nakatsuka T, Park JS, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999; 82:39–47.
Article25. Lima D, Avelino A, Coimbra A. Differential activation of c-fos in spinal neurones by distinct classes of noxious stimuli. Neuroreport. 1993; 4:747–750.
Article26. Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010; 115:505–514.
Article27. Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010; 12:133–148.
Article28. Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005; 144:940–952.
Article29. Brooks AC, Rickards KJ, Cunningham FM. Modulation of equine neutrophil adherence and migration by the annexin-1 derived N-terminal peptide, Ac2-26. Vet Immunol Immunopathol. 2012; 145:214–222.
Article30. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661.
Article31. Kim NR, Lee JW, Jun SR, Lee IJ, Lim SD, Yeom JS, et al. Effects of epidural TNF-α inhibitor injection: analysis of the pathological changes in a rat model of chronic compression of the dorsal root ganglion. Skeletal Radiol. 2012; 41:539–545.
Article32. Sielenkämper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000; 93:844–851.
Article33. Dirks J, Møiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002; 97:1591–1596.
Article34. Lavand'homme P. From preemptive to preventive analgesia: time to reconsider the role of perioperative peripheral nerve blocks? Reg Anesth Pain Med. 2011; 36:4–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Interactions between Intrathecal Gabapentin and MK801 or NBQX in Rat Formalin Test
- Preemptive Effect of Ketamine on Inflammatory Pain and Spinal c-fos Expression Induced by Formalin in the Rat
- Effect of Epidural Dexamethasone Injection for Back Pain after Lumbar Epidural Anesthesia
- The Effects of Milnacipran on Rat Pain Model
- Antinociceptive Effects of Intraperitoneal and Intrathecal Vitamin E in the Rat Formalin Test