Korean J Radiol.
2015 Apr;16(2):304-313. 10.3348/kjr.2015.16.2.304.
Fluid Retention Associated with Imatinib Treatment in Patients with Gastrointestinal Stromal Tumor: Quantitative Radiologic Assessment and Implications for Management
- Affiliations
-
- 1Department of Imaging, Dana-Farber Cancer Institute, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA. medimash@gmail.com
- 2Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- 3The Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA 02111, USA.
- KMID: 2070174
- DOI: http://doi.org/10.3348/kjr.2015.16.2.304
Abstract
OBJECTIVE
We aimed to describe radiologic signs and time-course of imatinib-associated fluid retention (FR) in patients with gastrointestinal stromal tumor (GIST), and its implications for management.
MATERIALS AND METHODS
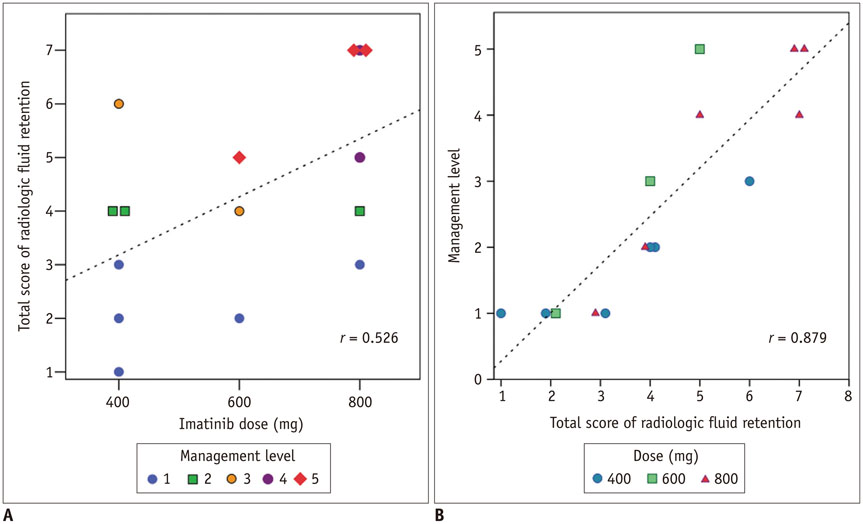
In this Institutional Review Board-approved, retrospective study of 403 patients with GIST treated with imatinib, 15 patients with imaging findings of FR were identified by screening radiology reports, followed by manual confirmation. Subcutaneous edema, ascites, pleural effusion, and pericardial effusion were graded on a four-point scale on CT scans; total score was the sum of these four scores.
RESULTS
The most common radiologic sign of FR was subcutaneous edema (15/15, 100%), followed by ascites (12/15, 80%), pleural effusion (11/15, 73%), and pericardial effusion (6/15, 40%) at the time of maximum FR. Two distinct types of FR were observed: 1) acute/progressive FR, characterized by acute aggravation of FR and rapid improvement after management, 2) intermittent/steady FR, characterized by occasional or persistent mild FR. Acute/progressive FR always occurred early after drug initiation/dose escalation (median 1.9 month, range 0.3-4.0 months), while intermittent/steady FR occurred at any time. Compared to intermittent/steady FR, acute/progressive FR was severe (median score, 5 vs. 2.5, p = 0.002), and often required drug-cessation/dose-reduction.
CONCLUSION
Two distinct types (acute/progressive and intermittent/steady FR) of imatinib-associated FR are observed and each type requires different management.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antineoplastic Agents/*adverse effects/therapeutic use
Ascites/pathology/radiography
Benzamides/*adverse effects/therapeutic use
Echocardiography/methods
Edema/pathology/radiography
Female
Gastrointestinal Stromal Tumors/drug therapy/pathology/*radiography
Gastrointestinal Tract/pathology/*radiography
Heart Failure/radiography
Humans
Male
Middle Aged
Molecular Targeted Therapy/*adverse effects
Pericardial Effusion/pathology/radiography
Peritoneal Neoplasms/diagnosis/radiography/secondary
Piperazines/*adverse effects/therapeutic use
Pleural Effusion/pathology/radiography
Pyrimidines/*adverse effects/therapeutic use
Radiology
Retrospective Studies
Tomography, X-Ray Computed
Antineoplastic Agents
Benzamides
Piperazines
Pyrimidines
Figure
Reference
-
1. Siddiqui MA, Scott LJ. Imatinib: a review of its use in the management of gastrointestinal stromal tumours. Drugs. 2007; 67:805–820.2. Thanopoulou E, Judson I. The safety profile of imatinib in CML and GIST: long-term considerations. Arch Toxicol. 2012; 86:1–12.3. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.4. Verweij J, van Oosterom A, Blay JY, Judson I, Rodenhuis S, van der Graaf W, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003; 39:2006–2011.5. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004; 364:1127–1134.6. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008; 26:626–632.7. Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006; 26:481–495.8. Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011; 37:75–88.9. Kim KW, Choi HJ, Kang S, Park SY, Jung DC, Cho JY, et al. The utility of multi-detector computed tomography in the diagnosis of malignant pleural effusion in the patients with ovarian cancer. Eur J Radiol. 2010; 75:230–235.10. Figueras J, Juncal A, Carballo J, Cortadellas J, Soler JS. Nature and progression of pericardial effusion in patients with a first myocardial infarction: relationship to age and free wall rupture. Am Heart J. 2002; 144:251–258.11. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–181.12. Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: definitions and clinical implications. Cancer. 2011; 117:688–697.13. Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004; 22:935–942.14. Ostro D, Lipton J. Unusual fluid retention with imatinib therapy for chronic myeloid leukemia. Leuk Lymphoma. 2007; 48:195–196.15. Van Glabbeke M, Verweij J, Casali PG, Simes J, Le Cesne A, Reichardt P, et al. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG). Eur J Cancer. 2006; 42:2277–2285.16. Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012; 136:483–489.17. Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006; 12:908–916.18. Verweij J, Casali PG, Kotasek D, Le Cesne A, Reichard P, Judson IR, et al. Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: analysis of EORTC-ISG-AGITG study 62005. Eur J Cancer. 2007; 43:974–978.19. Trent JC, Patel SS, Zhang J, Araujo DM, Plana JC, Lenihan DJ, et al. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer. 2010; 116:184–192.20. Heuchel R, Berg A, Tallquist M, Ahlén K, Reed RK, Rubin K, et al. Platelet-derived growth factor beta receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3' kinase signaling. Proc Natl Acad Sci U S A. 1999; 96:11410–11415.21. Pietras K, Ostman A, Sjöquist M, Buchdunger E, Reed RK, Heldin CH, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001; 61:2929–2934.22. Masiello D, Gorospe G 3rd, Yang AS. The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on dasatinib. J Hematol Oncol. 2009; 2:46.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor
- A Case of Imatinib-mesylate associated Hypersensitivity Pneumonitis
- Successful Endoscopic Resection of a Rectal Gastrointestinal Stromal Tumor Larger Than 5 cm
- Imatinib-induced hepatitis treated by corticosteroids in a patient with metastatic gastrointestinal stromal tumor
- Postoperative Cure for Metastatic Gastrointestinal Stromal Tumor