Korean J Gastroenterol.
2021 Oct;78(4):235-239. 10.4166/kjg.2021.097.
Successful Endoscopic Resection of a Rectal Gastrointestinal Stromal Tumor Larger Than 5 cm
- Affiliations
-
- 1Departments of Internal Medicine , Chosun University College of Medicine, Gwangju, Korea
- 2Departments of Pathology, Chosun University College of Medicine, Gwangju, Korea
- KMID: 2521498
- DOI: http://doi.org/10.4166/kjg.2021.097
Abstract
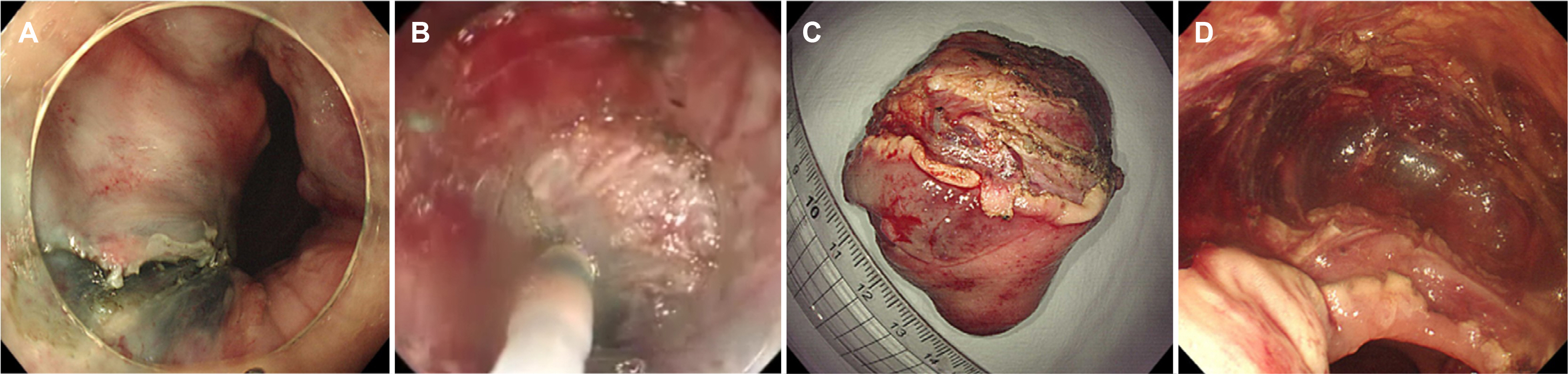
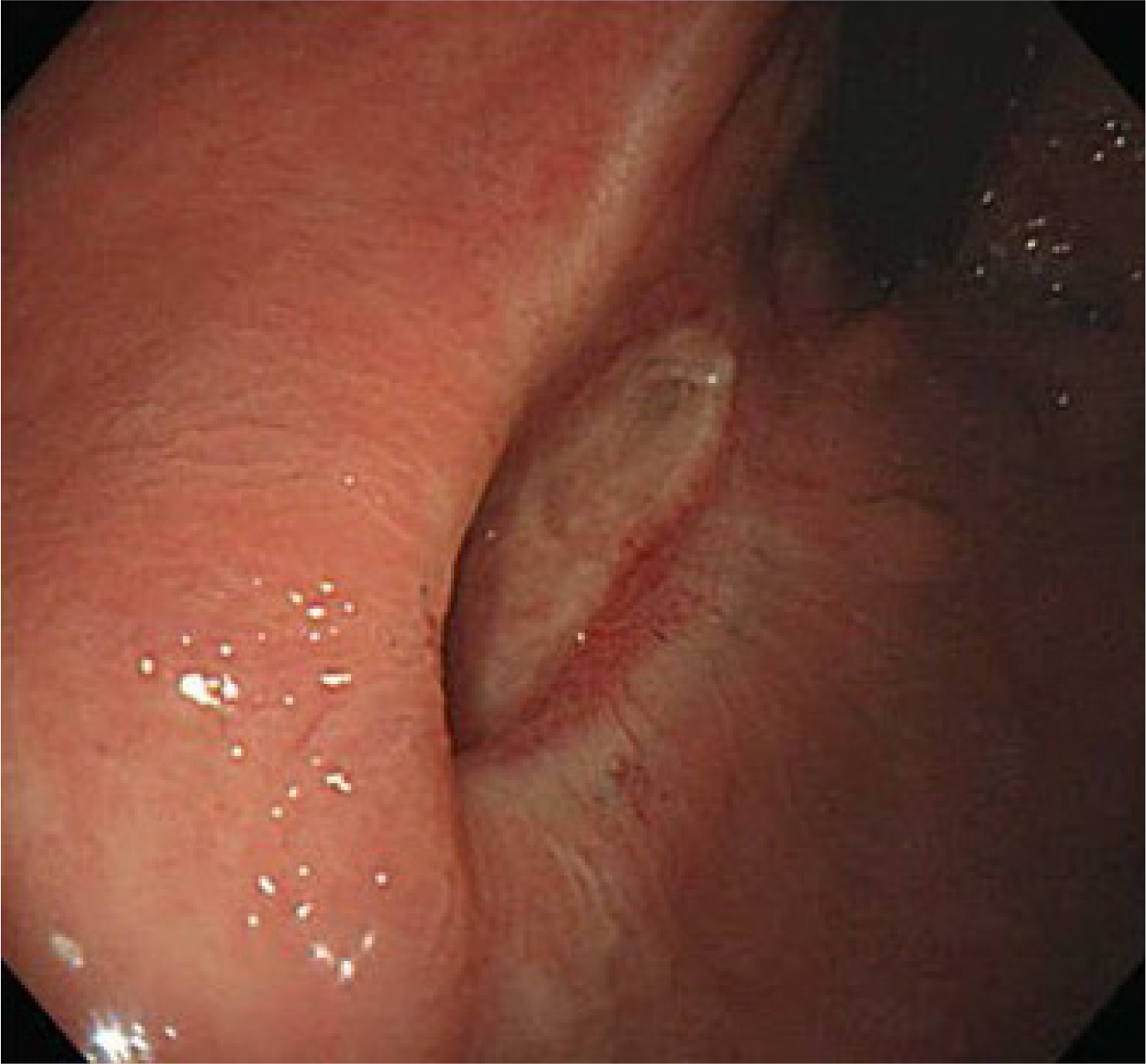
- Preoperative imatinib treatment for rectal gastrointestinal stromal tumors (GISTs) has been reported to reduce the tumor size and help preserve the anal sphincter function. On the other hand, preoperative imatinib may prevent an accurate assessment of the recurrent risk. The endoscopic resection of rectal GIST is rarely reported because of challenges that include securing the visual field and avoiding perforation. This paper reports a case in which a 5.5×4.0 cm sized rectal GIST was treated effectively by an endoscopic submucosal dissection (ESD) without preoperative imatinib. To date, the patient had no tumor recurrence or complications and is receiving adjuvant imatinib treatment. This case shows that ESD may be a good treatment option to preserve the anus in rectal GIST treatment.
Figure
Reference
-
1. Fletcher CD, Berman JJ, Corless C, et al. 2002; Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 33:459–465. DOI: 10.1053/hupa.2002.123545. PMID: 12094370.
Article2. von Mehren M, Randall RL, Benjamin RS, et al. 2018; Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 16:536–563. DOI: 10.6004/jnccn.2018.0025. PMID: 29752328.
Article3. Koo DH, Ryu MH, Kim KM, et al. 2016; Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 48:1155–1166. DOI: 10.4143/crt.2016.187. PMID: 27384163. PMCID: PMC5080813.
Article4. Blesius A, Cassier PA, Bertucci F, et al. 2011; Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer. 11:72. DOI: 10.1186/1471-2407-11-72. PMID: 21324142. PMCID: PMC3052196.
Article5. Eisenberg BL, Harris J, Blanke CD, et al. 2009; Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 99:42–47. DOI: 10.1002/jso.21160. PMID: 18942073. PMCID: PMC2606912.
Article6. Patrikidou A, Domont J, Chabaud S, et al. 2016; Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 52:173–180. DOI: 10.1016/j.ejca.2015.10.069. PMID: 26687836.
Article7. Han X, Xu J, Qiu H, Lin G. 2017; A novel curative treatment strategy for patients with lower grade rectal gastrointestinal stromal tumor: chemoreduction combined with transanal endoscopic microsurgery. J Laparoendosc Adv Surg Tech A. 27:579–585. DOI: 10.1089/lap.2017.0051. PMID: 28358587.
Article8. Liu Q, Zhong G, Zhou W, Lin G. 2017; Initial application of transanal endoscopic microsurgery for high-risk lower rectal gastrointestinal stromal tumor after imatinib mesylate neoadjuvant chemotherapy: a case report. Medicine (Baltimore). 96:e7538. DOI: 10.1097/MD.0000000000007538. PMID: 28723770. PMCID: PMC5521910.9. McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Aihara H. 2020; Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: a comparative systematic review and meta-analysis. Surg Endosc. 34:1688–1695. DOI: 10.1007/s00464-019-06945-1. PMID: 31292744.
Article10. Jung Y, Lee J, Cho JY, et al. 2018; Comparison of efficacy and safety between endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumor. Saudi J Gastroenterol. 24:115–121. DOI: 10.4103/sjg.SJG_440_17. PMID: 29637919. PMCID: PMC5900471.
Article11. Konuma H, Fu K, Konuma I, et al. 2011; A rectal GI stromal tumor completely resected with endoscopic submucosal dissection (with video). Gastrointest Endosc. 73:1322–1325. DOI: 10.1016/j.gie.2010.09.017. PMID: 21111411.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interpretation of Pathologic Margin after Endoscopic Resection of Gastrointestinal Stromal Tumor
- Three Cases of Endoscopic Mucosal Resection of Rectal Carcinoid Tumor by Band Ligation and the Snare Resection Technique
- Endoscopic Resection of Gastrointestinal Stromal Tumor: Is It Safe?
- Long-Term Outcomes after Endoscopic Treatment of Gastric Gastrointestinal Stromal Tumor
- Report 6 Cases of Rectal Carcinoid Tumor