Yonsei Med J.
2015 Mar;56(2):563-571. 10.3349/ymj.2015.56.2.563.
Differentially Expressed Proteins in Nitric Oxide-Stimulated NIH/3T3 Fibroblasts: Implications for Inhibiting Cancer Development
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Yonsei University, Seoul, Korea. kim626@yonsei.ac.kr
- 2Department of Food and Nutrition, Brain Korea 21 PLUS Project, College of Human Ecology, Yonsei University, Seoul, Korea. jwlim11@yonsei.ac.kr
- KMID: 2070039
- DOI: http://doi.org/10.3349/ymj.2015.56.2.563
Abstract
- PURPOSE
Recent evidence shows that nitric oxide (NO) may exhibit both pro-cancer and anti-cancer activities. The present study aimed to determine the differentially expressed proteins in NO-treated NIH/3T3 fibroblasts in order to investigate whether NO induces proteins with pro-cancer or anti-cancer effects.
MATERIALS AND METHODS
The cells were treated with 300 microM of an NO donor 3,3-bis-(aminoethyl)-1-hydroxy-2-oxo-1-triazene (NOC-18) for 12 h. The changed protein patterns, which were separated by two-dimensional electrophoresis using pH gradients of 4-7, were conclusively identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the peptide digests.
RESULTS
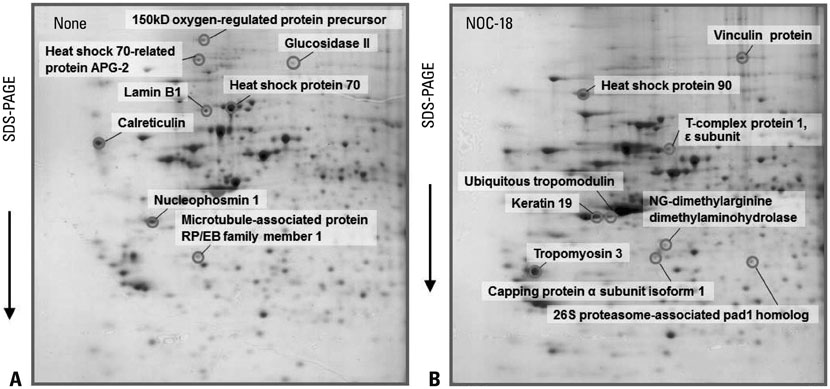
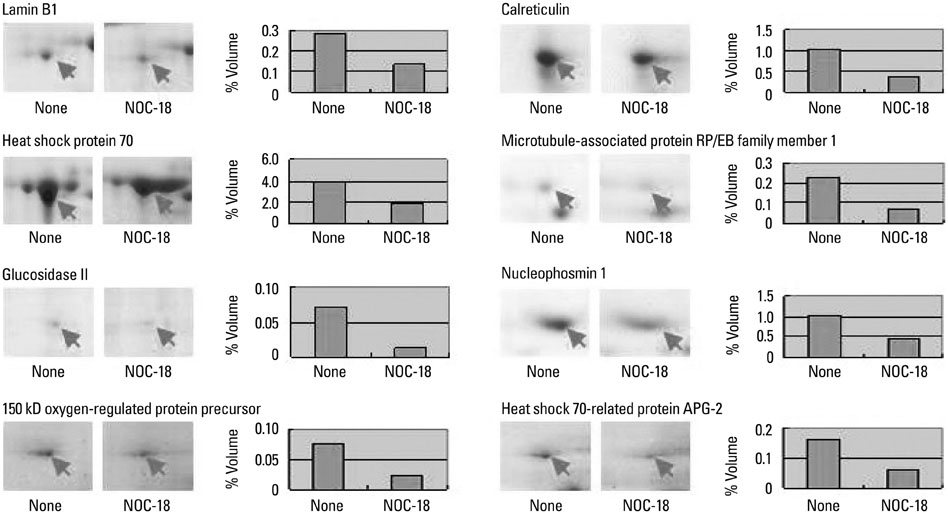
Seventeen differentially expressed proteins were identified in NOC-18-treated cells. Nine proteins [vinculin protein, keratin 19, ubiquitous tropomodulin, F-actin capping protein (alpha1 subunit), tropomyosin 3, 26S proteasome-associated pad1 homolog, T-complex protein 1 (epsilon subunit) N(G)-dimethylarginine dimethylaminohydrolase, and heat shock protein 90] were increased and eight proteins (heat shock protein 70, glucosidase II, lamin B1, calreticulin, nucleophosmin 1, microtubule-associated protein retinitis pigmentosa/end binding family member 1, 150 kD oxygen-regulated protein precursor, and heat shock 70-related protein albino or pale green 2) were decreased by NOC-18 in the cells. Thirteen proteins are related to the suppression of cancer cell proliferation, invasion, and metastasis while two proteins (heat shock protein 90 and N(G)-dimethylarginine dimethylaminohydrolase) are related to carcinogenesis. The functions of 150 kD oxygen-regulated protein precursor and T-complex protein 1 (epsilon subunit) are unknown in relation to carcinogenesis.
CONCLUSION
Most proteins differentially expressed by NOC-18 are involved in inhibiting cancer development.
Keyword
MeSH Terms
-
Animals
Electrophoresis, Gel, Two-Dimensional/*methods
Fibroblasts/*metabolism/pathology
HSP70 Heat-Shock Proteins
Humans
Mice
NIH 3T3 Cells
Neoplasms/*metabolism/pathology
Nitric Oxide Donors
Nitroso Compounds
Proteins/analysis/*metabolism
Proteomics/*methods
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
HSP70 Heat-Shock Proteins
Nitric Oxide Donors
Nitroso Compounds
Proteins
Figure
Reference
-
1. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–142.2. Beck KF, Eberhardt W, Frank S, Huwiler A, Messmer UK, Mühl H, et al. Inducible NO synthase: role in cellular signalling. J Exp Biol. 1999; 202(Pt 6):645–653.
Article3. Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991; 289:130–136.
Article4. Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol. 2005; 15:277–289.
Article5. Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007; 67:1407–1410.
Article6. Sugita H, Kaneki M, Furuhashi S, Hirota M, Takamori H, Baba H. Nitric oxide inhibits the proliferation and invasion of pancreatic cancer cells through degradation of insulin receptor substrate-1 protein. Mol Cancer Res. 2010; 8:1152–1163.
Article7. Carneiro ZA, Biazzotto JC, Alexiou AD, Nikolaou S. Nitric oxide photorelease from a trinuclear ruthenium nitrosyl complex and its in vitro cytotoxicity against melanoma cells. J Inorg Biochem. 2014; 134:36–38.
Article8. Brantley EC, Guo L, Zhang C, Lin Q, Yokoi K, Langley RR, et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol. 2010; 3:380–388.
Article9. Patel HJ, Belvisi MG, Donnelly LE, Yacoub MH, Chung KF, Mitchell JA. Constitutive expressions of type I NOS in human airway smooth muscle cells: evidence for an antiproliferative role. FASEB J. 1999; 13:1810–1816.
Article10. Seo JY, Yu JH, Lim JW, Mukaida N, Kim H. Nitric oxide-induced IL-8 expression is mediated by NF-kappaB and AP-1 in gastric epithelial AGS cells. J Physiol Pharmacol. 2009; 60:Suppl 7. 101–106.11. Lim JW, Kim H, Kim KH. NF-kappaB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Radic Biol Med. 2001; 31:355–366.
Article12. Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008; 16:624–630.
Article13. Yu JH, Yun SY, Lim JW, Kim H, Kim KH. Proteome analysis of rat pancreatic acinar cells: implication for cerulein-induced acute pancreatitis. Proteomics. 2003; 3:2446–2453.
Article14. Faure O, Graff-Dubois S, Bretaudeau L, Derré L, Gross DA, Alves PM, et al. Inducible Hsp70 as target of anticancer immunotherapy: Identification of HLA-A*0201-restricted epitopes. Int J Cancer. 2004; 108:863–870.
Article15. Gabai VL, Yaglom JA, Waldman T, Sherman MY. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009; 29:559–569.
Article16. Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002; 21:4411–4419.
Article17. Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem. 2008; 283:2031–2041.
Article18. Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013; 19:347–365.
Article19. Ma L, Sato F, Sato R, Matsubara T, Hirai K, Yamasaki M, et al. Dual targeting of heat shock proteins 90 and 70 promotes cell death and enhances the anticancer effect of chemotherapeutic agents in bladder cancer. Oncol Rep. 2014; 31:2482–2492.
Article20. Guttmann DM, Koumenis C. The heat shock proteins as targets for radiosensitization and chemosensitization in cancer. Cancer Biol Ther. 2011; 12:1023–1031.
Article21. Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002; 32:269–278.
Article22. Hisaoka M, Matsuyama A, Nakamoto M. Aberrant calreticulin expression is involved in the dedifferentiation of dedifferentiated liposarcoma. Am J Pathol. 2012; 180:2076–2083.
Article23. Pfister AS, Hadjihannas MV, Röhrig W, Schambony A, Behrens J. Amer2 protein interacts with EB1 protein and adenomatous polyposis coli (APC) and controls microtubule stability and cell migration. J Biol Chem. 2012; 287:35333–35340.
Article24. Tamura N, Draviam VM. Microtubule plus-ends within a mitotic cell are 'moving platforms' with anchoring, signalling and force-coupling roles. Open Biol. 2012; 2:120132.
Article25. Morrison EE. The APC-EB1 interaction. Adv Exp Med Biol. 2009; 656:41–50.
Article26. Kim MJ, Yun HS, Hong EH, Lee SJ, Baek JH, Lee CW, et al. Depletion of end-binding protein 1 (EB1) promotes apoptosis of human non-small-cell lung cancer cells via reactive oxygen species and Bax-mediated mitochondrial dysfunction. Cancer Lett. 2013; 339:15–24.
Article27. Sun S, Xu MZ, Poon RT, Day PJ, Luk JM. Circulating Lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. J Proteome Res. 2010; 9:70–78.
Article28. Li L, Du Y, Kong X, Li Z, Jia Z, Cui J, et al. Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clin Cancer Res. 2013; 19:4651–4661.
Article29. Suradej B, Pata S, Kasinrerk W, Cressey R. Glucosidase II exhibits similarity to the p53 tumor suppressor in regards to structure and behavior in response to stress signals: a potential novel cancer biomarker. Oncol Rep. 2013; 30:2511–2519.
Article30. Wong JC, Hasan MR, Rahman M, Yu AC, Chan SK, Schaeffer DF, et al. Nucleophosmin 1, upregulated in adenomas and cancers of the colon, inhibits p53-mediated cellular senescence. Int J Cancer. 2013; 133:1567–1577.
Article31. Deng WH, Chen C, Wang WX, Yu J, Li JY, Liu L. Effects of ORP150 on appearance and function of pancreatic beta cells following acute necrotizing pancreatitis. Pathol Res Pract. 2011; 207:370–376.
Article32. Li C, Liu D, Yuan Y, Huang S, Shi M, Tao K, et al. Overexpression of Apg-2 increases cell proliferation and protects from oxidative damage in BaF3-BCR/ABL cells. Int J Oncol. 2010; 36:899–904.
Article33. Gotoh K, Nonoguchi K, Higashitsuji H, Kaneko Y, Sakurai T, Sumitomo Y, et al. Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett. 2004; 560:19–24.
Article34. Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol. 2011; 90:157–163.
Article35. Goldmann WH, Auernheimer V, Thievessen I, Fabry B. Vinculin, cell mechanics and tumour cell invasion. Cell Biol Int. 2013; 37:397–405.
Article36. Kostyukova AS. Tropomodulins and tropomodulin/tropomyosin interactions. Cell Mol Life Sci. 2008; 65:563–569.
Article37. Franzén B, Linder S, Uryu K, Alaiya AA, Hirano T, Kato H, et al. Expression of tropomyosin isoforms in benign and malignant human breast lesions. Br J Cancer. 1996; 73:909–913.
Article38. Zheng Q, Safina A, Bakin AV. Role of high-molecular weight tropomyosins in TGF-beta-mediated control of cell motility. Int J Cancer. 2008; 122:78–90.
Article39. Lim BH, Cho BI, Kim YN, Kim JW, Park ST, Lee CW. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp Mol Med. 2006; 38:455–465.
Article40. Lee YJ, Jeong SH, Hong SC, Cho BI, Ha WS, Park ST, et al. Prognostic value of CAPZA1 overexpression in gastric cancer. Int J Oncol. 2013; 42:1569–1577.
Article41. Schoenfeld A, Luqmani Y, Sinnett HD, Shousha S, Coombes RC. Keratin 19 mRNA measurement to detect micrometastases in lymph nodes in breast cancer patients. Br J Cancer. 1996; 74:1639–1642.
Article42. Ju JH, Yang W, Lee KM, Oh S, Nam K, Shim S, et al. Regulation of cell proliferation and migration by keratin19-induced nuclear import of early growth response-1 in breast cancer cells. Clin Cancer Res. 2013; 19:4335–4346.
Article43. Guenther MG, Yu J, Kao GD, Yen TJ, Lazar MA. Assembly of the SMRT-histone deacetylase 3 repression complex requires the TCP-1 ring complex. Genes Dev. 2002; 16:3130–3135.
Article44. Nikawa J, Kimura M. A novel function of the human chaperonin CCT epsilon subunit in yeast. Biosci Biotechnol Biochem. 2012; 76:199–201.
Article45. Lagadec C, Vlashi E, Bhuta S, Lai C, Mischel P, Werner M, et al. Tumor cells with low proteasome subunit expression predict overall survival in head and neck cancer patients. BMC Cancer. 2014; 14:152.
Article46. Boult JK, Walker-Samuel S, Jamin Y, Leiper JM, Whitley GS, Robinson SP. Active site mutant dimethylarginine dimethylaminohydrolase 1 expression confers an intermediate tumour phenotype in C6 gliomas. J Pathol. 2011; 225:344–352.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Candida spp.- Induced Cytokine Gene Expression on Mouse Peritoneal Macrophages and NIH 3T3 Fibroblasts
- Nitric Oxide: The Pathophysiological Roles and Clinical Implications in Circulatory System
- Inhibitory Effect of Esculetin on the Inducuble Nitric Oxide Synthase Expression in TNF-stimulated 3T3-L1 Adipocytes
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells
- Studies on the Cytotoxicity and Antineoplastic Activity of Methyl Gallate