Yonsei Med J.
2015 Mar;56(2):497-502. 10.3349/ymj.2015.56.2.497.
A Melting Method for RNA Extraction from the Mucosal Membrane of the Mouse Middle Ear
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea. jychoi@yuhs.ac
- KMID: 2070030
- DOI: http://doi.org/10.3349/ymj.2015.56.2.497
Abstract
- PURPOSE
There is much confusion surrounding the methods of RNA extraction from the middle ear mucosa of mice. In this study, we worked to develop a "melting method," which is faster, purer, and more reliable than other methods in common use.
MATERIALS AND METHODS
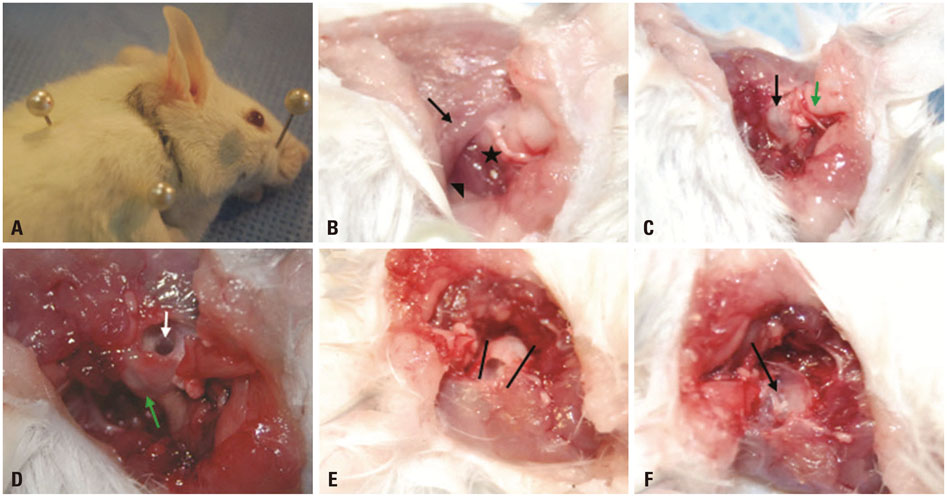
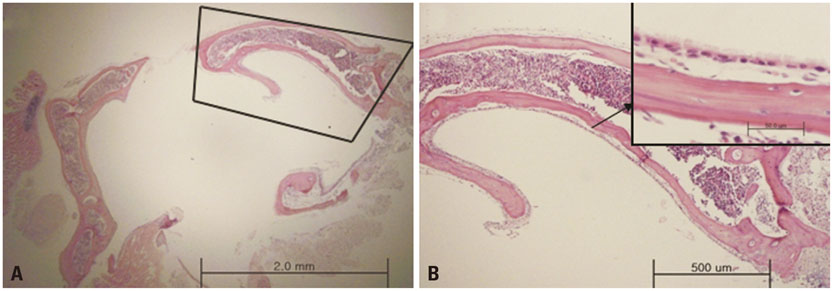
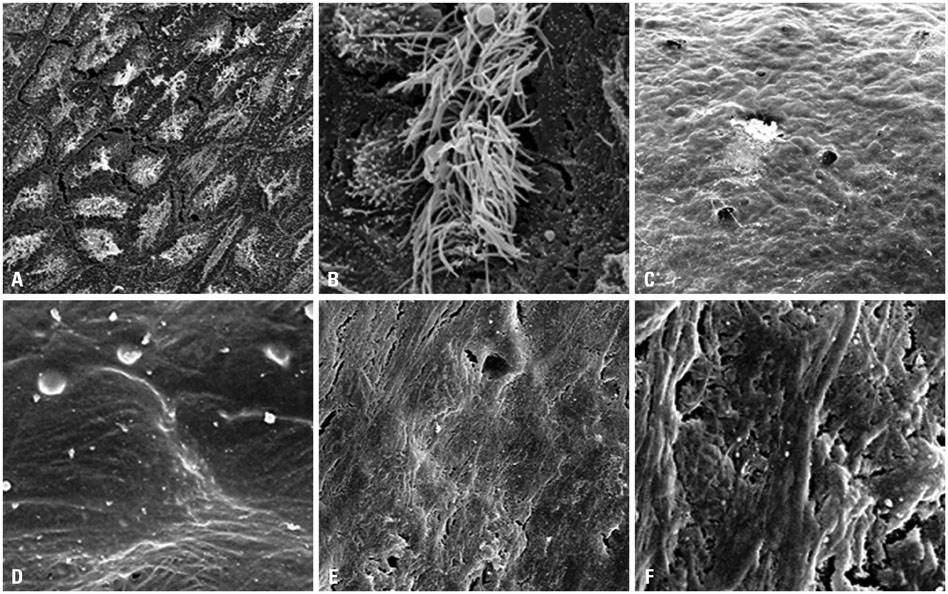
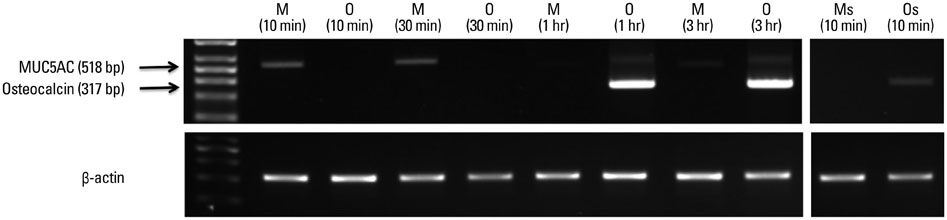
Thirty-two ears were used for this study. Light microscopy with hematoxylin-eosin staining of the bullae, scanning electron microscopy (SEM), spectrophotometer analysis, and reverse transcription polymerase chain reaction were performed before and after melting the half lateral bullae, which were detached from the temporal bone by using a lateral retroauricular approach.
RESULTS
Each resected half bulla contained a well distributed mucosal membrane. After a TRIzol melting duration of 10-30 minutes, only mucosal marker (MUC5AC) was expressed without bony marker (total osteocalcin). The same results were determined from SEM.
CONCLUSION
This melting method, compared with stripping and irrigation methods, is effective and offers an easier, more robust approach to extracting RNA from the middle ear mucosal membranes of mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Wysocki J. Topographical anatomy and measurements of selected parameters of the rat temporal bone. Folia Morphol (Warsz). 2008; 67:111–119.2. Sichel JY, Plotnik M, Cherny L, Sohmer H, Elidan J. Surgical anatomy of the ear of the fat sand rat. J Otolaryngol. 1999; 28:217–222.3. Dogru S, Haholu A, Gungor A, Kucukodaci Z, Cincik H, Ozdemir T, et al. Histologic analysis of the effects of three different support materials within rat middle ear. Otolaryngol Head Neck Surg. 2009; 140:177–182.
Article4. Nell MJ, Grote JJ. Structural changes in the rat middle ear mucosa due to endotoxin and eustachian tube obstruction. Eur Arch Otorhinolaryngol. 1999; 256:167–172.
Article5. Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006; 27:126–139.
Article6. Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994; 269:1183–1190.
Article7. Kerschner JE, Li J, Tsushiya K, Khampang P. Mucin gene expression and mouse middle ear epithelium. Int J Pediatr Otorhinolaryngol. 2010; 74:864–868.
Article8. Judkins RF, Li H. Surgical anatomy of the rat middle ear. Otolaryngol Head Neck Surg. 1997; 117:438–447.
Article9. Cayé-Thomasen P, Hermansson A, Tos M, Prellner K. Increased secretory capacity of the middle ear mucosa after acute otitis media caused by Haemophilus influenzae type B. Otolaryngol Head Neck Surg. 1997; 117(3 Pt 1):263–267.10. Palacios SD, Pak K, Rivkin AZ, Kayali AG, Austen D, Aletsee C, et al. Role of p38 mitogen-activated protein kinase in middle ear mucosa hyperplasia during bacterial otitis media. Infect Immun. 2004; 72:4662–4667.
Article11. Chen YP, Tong HH, James , Demaria TF. Detection of mucin gene expression in normal rat middle ear mucosa by reverse transcriptase-polymerase chain reaction. Acta Otolaryngol. 2001; 121:45–51.
Article12. Furukawa M, Ebmeyer J, Pak K, Austin DA, Melhus A, Webster NJ, et al. Jun N-terminal protein kinase enhances middle ear mucosal proliferation during bacterial otitis media. Infect Immun. 2007; 75:2562–2571.
Article13. Pinilla M, Ramírez-Camacho R, Jorge E, Trinidad A, Vergara J. Ventral approach to the rat middle ear for otologic research. Otolaryngol Head Neck Surg. 2001; 124:515–517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of beta Defensins in the Human Middle Ear Mucosa
- The Effects of Antibiotics and Steroid on the Middle Ear Mucosa in the Rats with Experimental Acute Otitis Media
- Cholesterol Granuloma without Tympanic Membrane Perforation
- Tympanic Membrane Perforation Due to Metal Spark in a Welder
- A Case of Intratympanic Membrane Congenital Cholesteatoma