Pediatr Gastroenterol Hepatol Nutr.
2015 Sep;18(3):187-192. 10.5223/pghn.2015.18.3.187.
Diagnostic Value of Ceruloplasmin in the Diagnosis of Pediatric Wilson's Disease
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. seakhee.oh@amc.seoul.kr
- 2Medical Genetics Center, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2068796
- DOI: http://doi.org/10.5223/pghn.2015.18.3.187
Abstract
- PURPOSE
Measurement of serum ceruloplasmin level is the first step in screening for Wilson's disease (WD). Despite the rarity of WD in the general population, ceruloplasmin levels are routinely measured through hepatitis screening in both adults and children. Herein, we evaluated the diagnostic value of ceruloplasmin for the diagnosis of WD among children with hepatitis.
METHODS
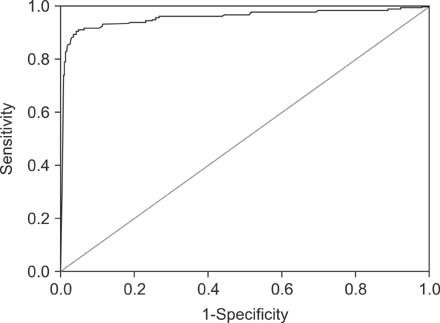
We retrospectively reviewed data on serum ceruloplasmin levels measured as a serologic marker for patients with hepatitis at Asan Medical Center (Seoul, Korea) between from January 2004 to November 2013. The diagnosis of WD was confirmed by the identification of pathogenic variants in the ATP7B gene. To determine the diagnostic accuracy of ceruloplasmin, receiver operation characteristic (ROC) curves were constructed and the area under curve (AUC) were calculated.
RESULTS
Measurements of serum ceruloplasmin were performed in 2,834 children who had hepatitis. Among these, 181 (6.4%) children were diagnosed with WD. The sensitivity, specificity, and accuracy of a ceruloplasmin level of <20 mg/dL in the discrimination of WD were 93.4%, 84.2%, and 84.8%, respectively. In this study, 418 (14.7%) false-positive cases and 12 (0.4%) false-negative cases were noted. Using a ROC curve, a ceruloplasmin level of < or =16.6 mg/dL showed the highest AUC value (0.956) with a sensitivity of 91.2%, a specificity of 94.9%, and an accuracy of 94.7%.
CONCLUSION
The measurement of serum ceruloplasmin was frequently used for the screening of WD in children, despite a low positive rate. The diagnostic value of ceruloplasmin may be strengthened by adopting a new lower cut-off level.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Multiplex Ligation-dependent Probe Amplification Analysis Subsequent to Direct DNA Full Sequencing for Identifying ATP7B Mutations and Phenotype Correlations in Children with Wilson Disease
Jung Ok Shim, Hye Ran Yang, Jin Soo Moon, Ju Young Chang, Jae Sung Ko, Sung Sup Park, Jeong Kee Seo
J Korean Med Sci. 2018;33(26):. doi: 10.3346/jkms.2018.33.e177.
Reference
-
1. Litwin T, Członkowska A. Wilson disease-factors affecting clinical presentation. Neurol Neurochir Pol. 2013; 47:161–169.2. Manolaki N, Nikolopoulou G, Daikos GL, Panagiotakaki E, Tzetis M, Roma E, et al. Wilson disease in children: analysis of 57 cases. J Pediatr Gastroenterol Nutr. 2009; 48:72–77.
Article3. Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010; 24:531–539.
Article4. Endo F, Taketa K, Nakamura K, Awata H, Tanoue A, Eda Y, et al. Measurement of blood holoceruloplasmin by EIA using a mouse monoclonal antibody directed to holoceruloplasmin. Implication for mass screening of Wilson disease. J Inherit Metab Dis. 1994; 17:616–620.
Article5. Ohura T, Abukawa D, Shiraishi H, Yamaguchi A, Arashima S, Hiyamuta S, et al. Pilot study of screening for Wilson disease using dried blood spots obtained from children seen at outpatient clinics. J Inherit Metab Dis. 1999; 22:74–80.
Article6. Yamaguchi Y, Aoki T, Arashima S, Ooura T, Takada G, Kitagawa T, et al. Mass screening for Wilson's disease: results and recommendations. Pediatr Int. 1999; 41:405–408.
Article7. Seo JK. Diagnosis of Wilson disease in young children: molecular genetic testing and a paradigm shift from the laboratory diagnosis. Pediatr Gastroenterol Hepatol Nutr. 2012; 15:197–209.
Article8. Ala A, Borjigin J, Rochwarger A, Schilsky M. Wilson disease in septuagenarian siblings: raising the bar for diagnosis. Hepatology. 2005; 41:668–670.
Article9. Beyersdorff A, Findeisen A. Morbus Wilson: case report of a two-year-old child as first manifestation. Scand J Gastroenterol. 2006; 41:496–497.
Article10. Schoen RE, Sternlieb I. Clinical aspects of Wilson's disease. Am J Gastroenterol. 1990; 85:1453–1457.11. Mak CM, Lam CW, Tam S. Diagnostic accuracy of serum ceruloplasmin in Wilson disease: determination of sensitivity and specificity by ROC curve analysis among ATP7B-genotyped subjects. Clin Chem. 2008; 54:1356–1362.
Article12. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77.
Article13. Hahn SH. Population screening for Wilson's disease. Ann N Y Acad Sci. 2014; 1315:64–69.
Article14. Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006; 148:652–658.
Article15. Korman JD, Volenberg I, Balko J, Webster J, Schiodt FV, Squires RH Jr, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008; 48:1167–1174.
Article16. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013; 126:926.e1–926.e5.
Article17. Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003; 23:139–142.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wilson's Disease: Report of a Case with Comprehensive Review of the Previously Reported Cases in Korean Literature
- Wilson's Disease: Report of a Case with Comprehensive Review of the Previously Reported Cases in Korean Literature
- The Study of the Initial Presentations of Wilson Disease at Diagonosis
- Development of a Screening Kit for Early Diagnosis and Prevention of Wilson's Disease
- The Challenges of Diagnosing and Following Wilson Disease in the Presence of Proteinuria