J Bacteriol Virol.
2015 Sep;45(3):215-227. 10.4167/jbv.2015.45.3.215.
Characterization of Endoplasmic Reticulum Stress and Apoptosis in Macrophages Infected with Mycobacterium tuberculosis Isolates from Korea Patients
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon, Korea. songch@cnu.ac.kr
- 2Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3Research Institute for Medical Sciences, College of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 2068736
- DOI: http://doi.org/10.4167/jbv.2015.45.3.215
Abstract
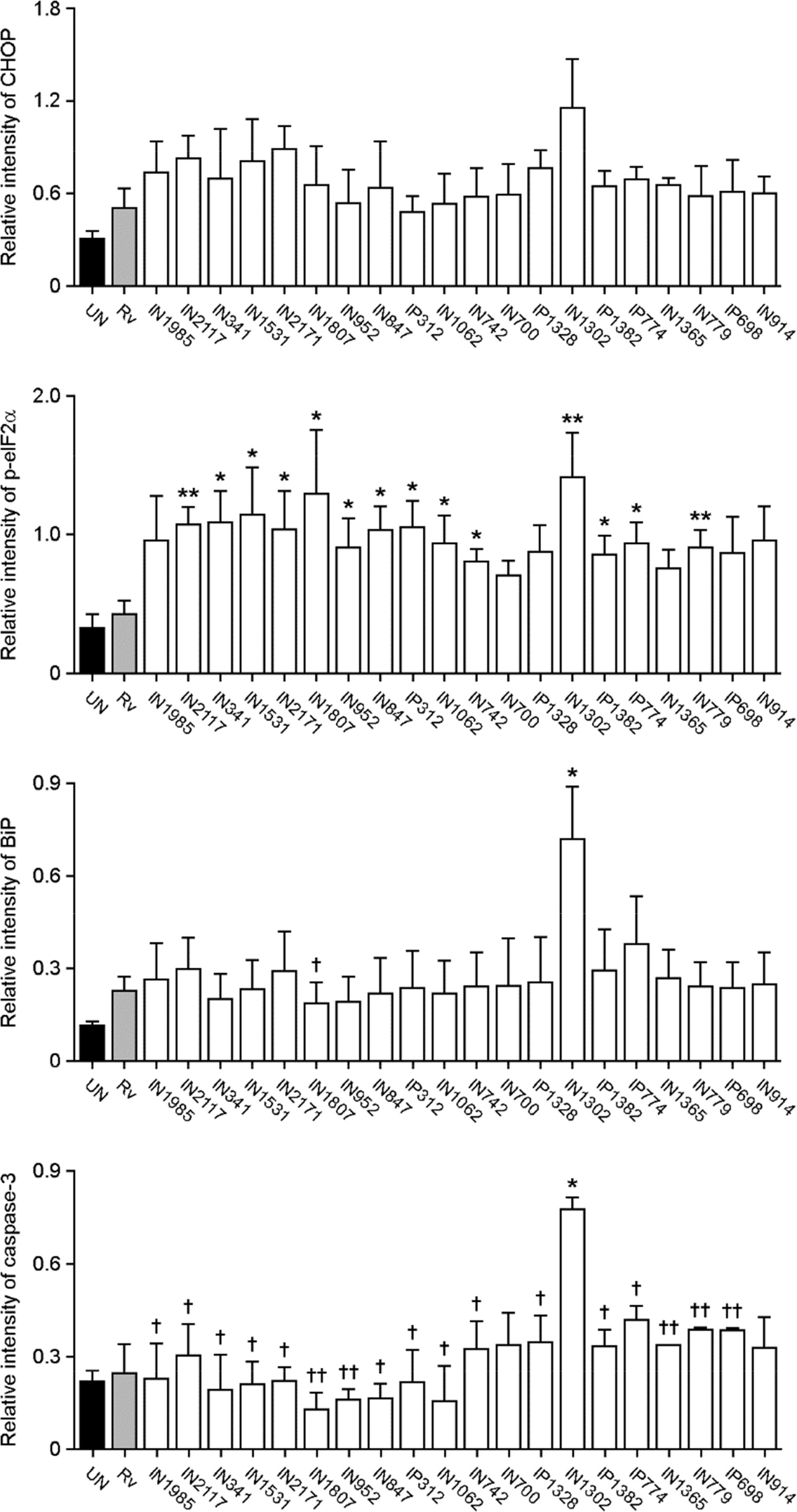
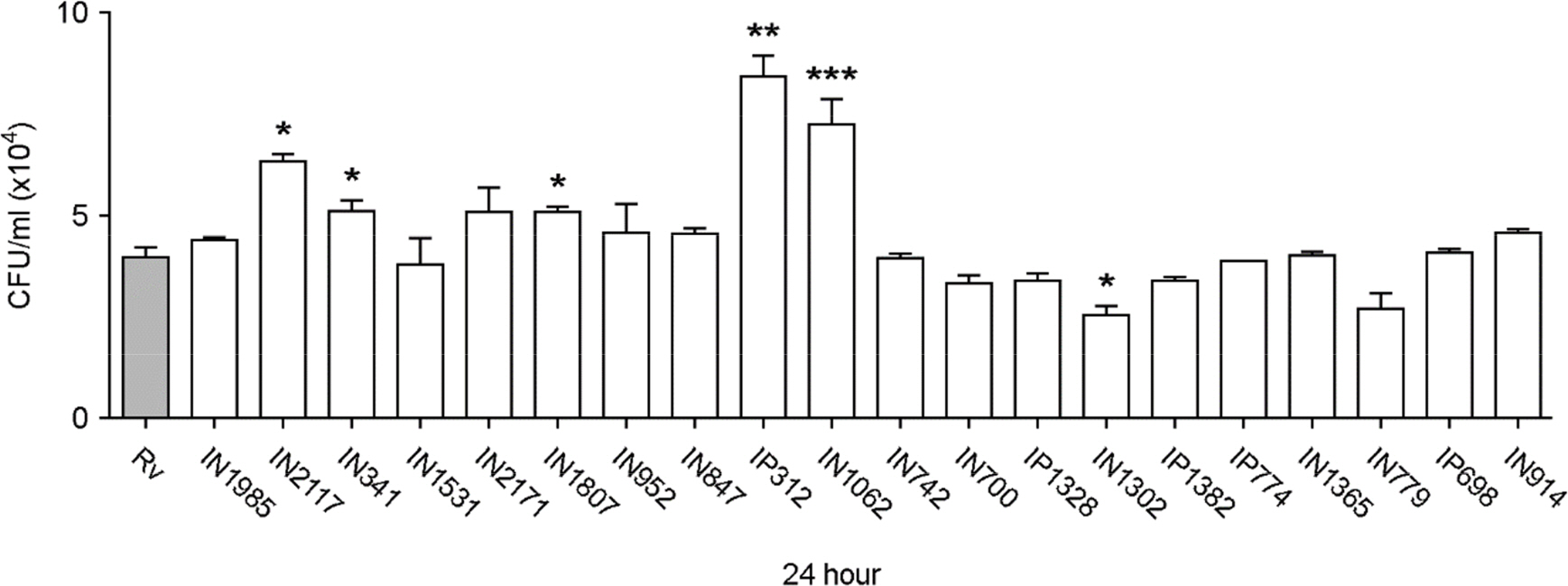
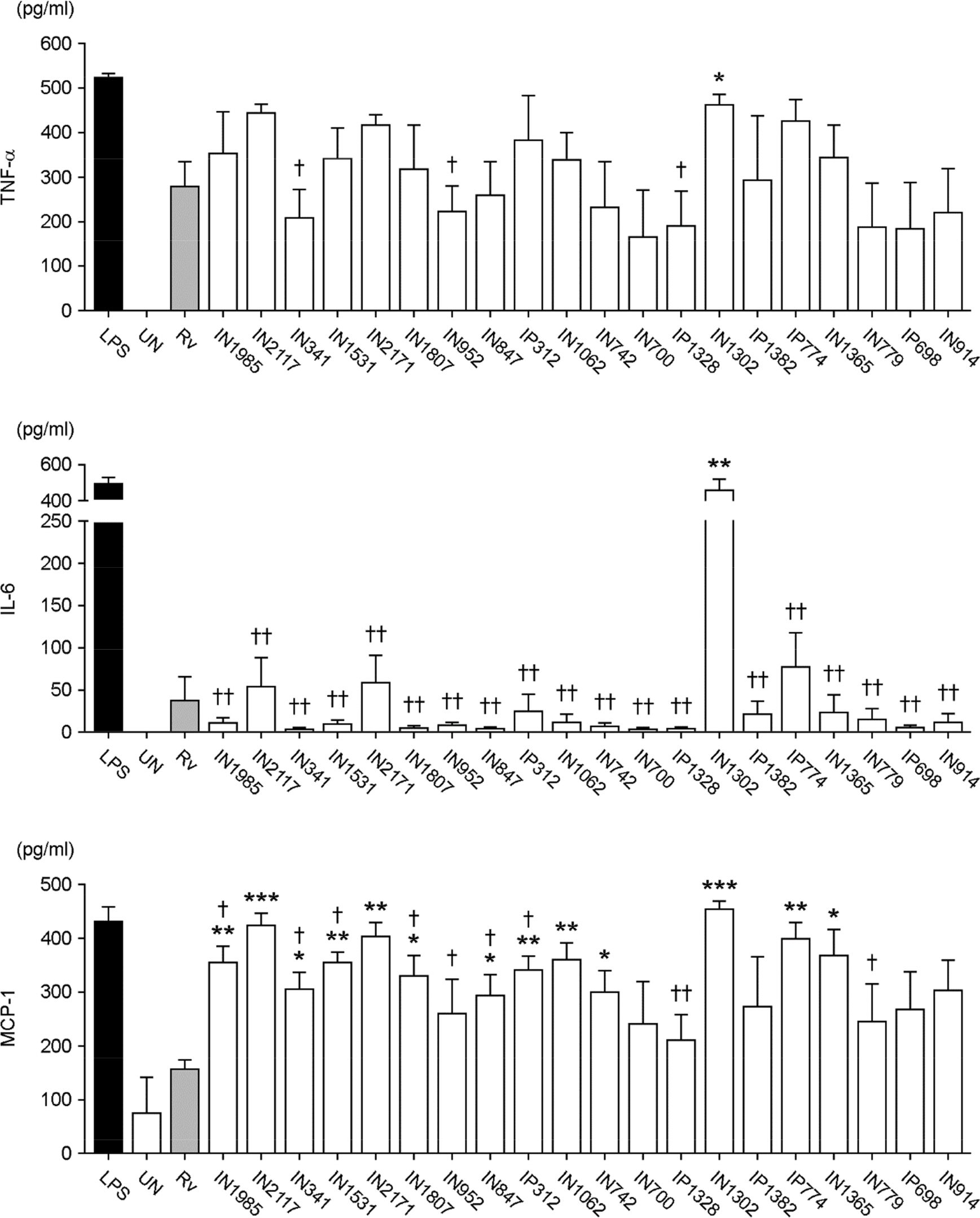
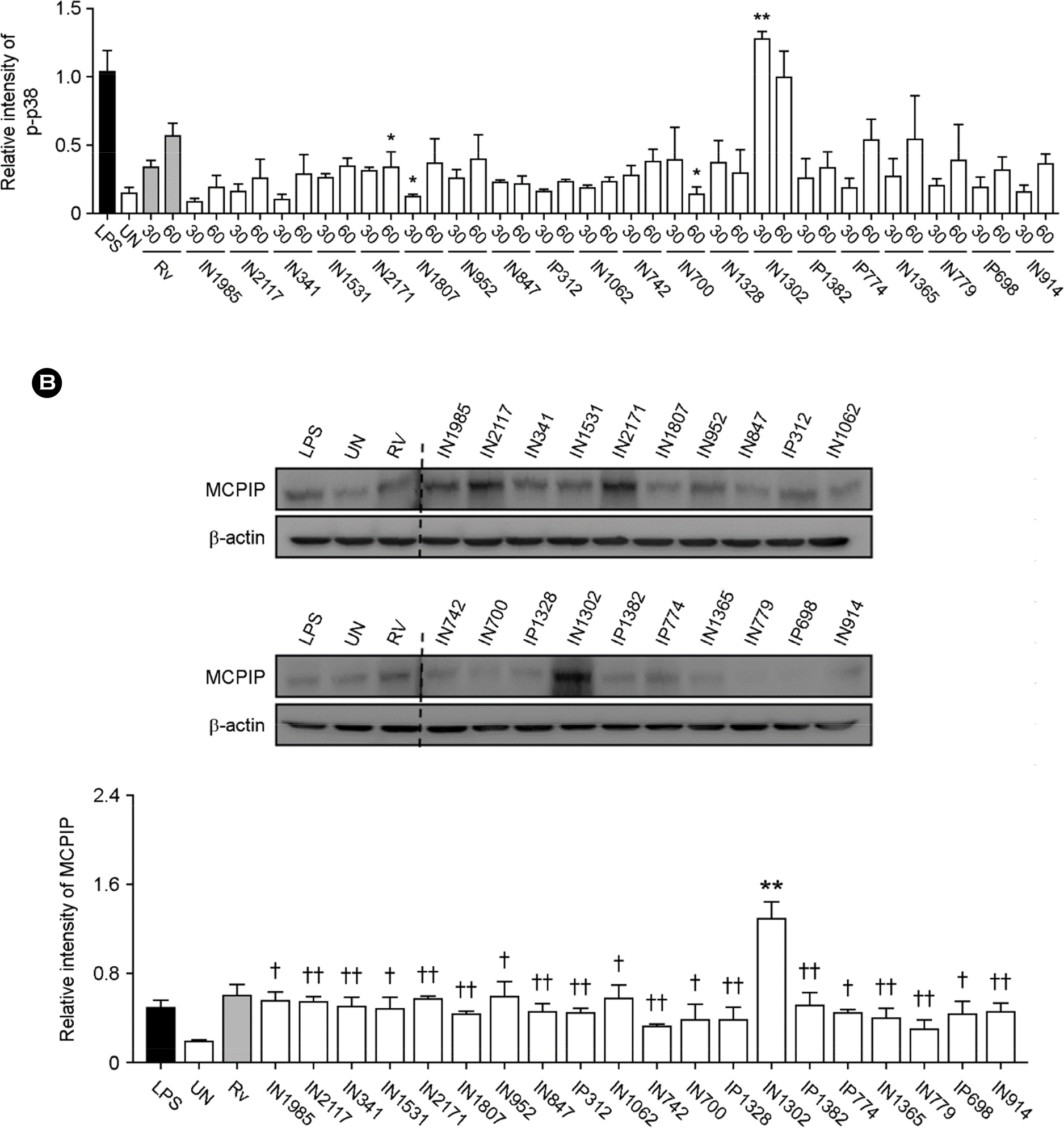
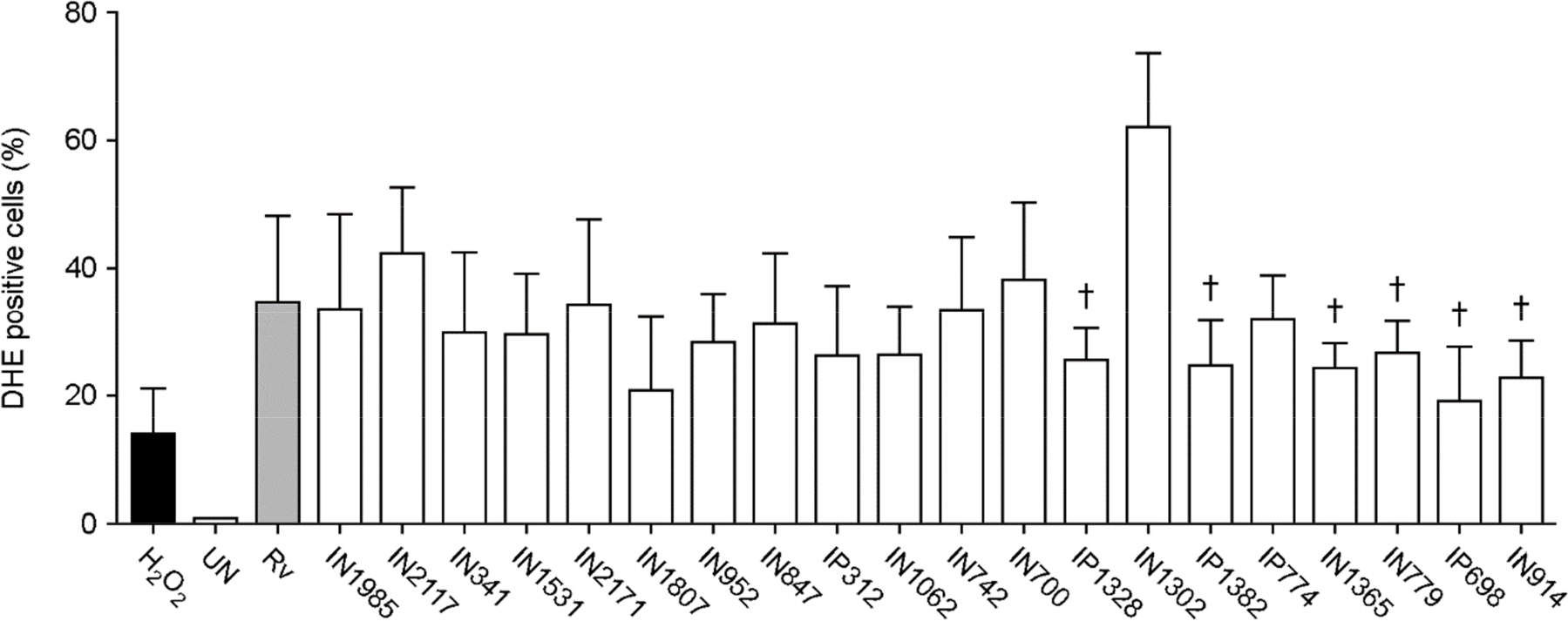
- Apoptosis is an important host defense mechanism against mycobacterial infection. Recent reports suggest that links between apoptosis and endoplasmic reticulum (ER) stress are critical for the regulation of mycobacterial survival; however, the exact regulatory mechanisms are not well known. In this study, we isolated 20 Mycobacterium tuberculosis (Mtb) clinical strains from Korean patients and examined ER stress-mediated apoptosis in Mtb-infected macrophages. Most Mtb strains increased the rates of apoptosis and production of ER stress-sensing molecules in mouse macrophages, similar to Mtb H37Rv infection. Moreover, the intracellular survival of Mtb clinical isolates in macrophages was similar to that of H37Rv. Our data suggest that infection with Mtb downregulated MCP-1 and MCPIP. The regulation of MCPIP may decrease ROS production, leading to a reduction in ER stress-mediated apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
1). van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002; 15:294–309.2). Zumla A, George A, Sharma V, Herbert RH, Baroness Masham of Ilton , Oxley A, et al. The WHO 2014 Global tuberculosis report–further to go. Lancet Glob Health. 2015; 3:e10–2.
Article3). Faherty CS, Maurelli AT. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008; 16:173–80.
Article4). Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 2003; 5:99–109.5). Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005; 280:9058–64.6). Lee HC, Goodman JL. Anaplasma phagocytophilum causes global induction of antiapoptosis in human neutrophils. Genomics. 2006; 88:496–503.7). Schmid MC, Scheidegger F, Dehio M, Balmelle-Devaux N, Schulein R, Guye P, et al. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog. 2006; 2:e115.
Article8). Joshi SG, Francis CW, Silverman DJ, Sahni SK. NF-kappaB activation suppresses host cell apoptosis during Rickettsia rickettsii infection via regulatory effects on intracellular localization or levels of apoptogenic and anti-apoptotic proteins. FEMS Microbiol Lett. 2004; 234:333–41.9). Zhang JZ, Sinha M, Luxon BA, Yu XJ. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004; 72:498–507.10). Clark CS, Maurelli AT. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect Immun. 2007; 75:2531–9.11). Divangahi M, Behar SM, Remold H. Dying to Live: How the Death Modality of the Infected Macrophage Affects Immunity to Tuberculosis. Divangahi M, editor. The New Paradigm of Immunity to Tuberculosis:. Springer New York;2013. p. 103–20.
Article12). Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000; 164:2016–20.13). Song CH. Endoplasmic Reticulum Stress Responses and Apoptosis. J Bacteriol Virol. 2012; 42:196–202.
Article14). Choi HH, Shin DM, Kang G, Kim KH, Park JB, Hur GM, et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett. 2010; 584:2445–54.15). Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006; 7:880–5.
Article16). Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002; 7:335–45.17). Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005; 74:739–89.
Article18). Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003; 23:1292–303.19). Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014; 8:213.
Article20). Lee SY, Lee MS, Cherla RP, Tesh VL. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008; 10:770–80.
Article21). Pillich H, Loose M, Zimmer KP, Chakraborty T. Activation of the unfolded protein response by Listeria monocytogenes. Cell Microbiol. 2012; 14:949–64.22). Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002; 76:4162–71.23). Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, Jo EK, et al. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One. 2011; 6:e28531.24). Choi JA, Lim YJ, Cho SN, Lee JH, Jeong JA, Kim EJ, et al. Mycobacterial HBHA induces endoplasmic reticulum stress-mediated apoptosis through the generation of reactive oxygen species and cytosolic Ca2+ in murine macrophage RAW 264.7 cells. Cell Death Dis. 2013; 4:e957.
Article25). Lim YJ, Choi JA, Lee JH, Choi CH, Kim HJ, Song CH. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis. 2015; 20:358–70.26). Lee J, Hartman M, Kornfeld H. Macrophage Apoptosis in Tuberculosis. Yonsei Med J. 2009; 50:1–11.
Article27). Hao X, Yao A, Gong J, Zhu W, Li N, Li J. Berberine ameliorates pro-inflammatory cytokine-induced endoplasmic reticulum stress in human intestinal epithelial cells in vitro. Inflammation. 2012; 35:841–9.28). Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012; 110:174–89.
Article29). Younce C, Kolattukudy P. MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem. 2012; 30:307–20.
Article30). Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012; 24:1548–55.
Article31). Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, et al. Variation among Genome Sequences of H37Rv Strains of Mycobacterium tuberculosis from Multiple Laboratories. J Bacteriol. 2010; 192:3645–53.32). Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, Gan HX, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011; 4:279–87.33). Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010; 8:668–74.34). Bezuidenhout J, Roberts T, Muller L, van Helden P, Walzl G. Pleural tuberculosis in patients with early HIV infection is associated with increased TNF-alpha expression and necrosis in granulomas. PLoS One. 2009; 4:e4228.
Article35). Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997; 158:4320–7.36). Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013; 153:521–34.
Article37). Bocchino M, Galati D, Sanduzzi A, Colizzi V, Brunetti E, Mancino G. Role of mycobacteria-induced monocyte/macrophage apoptosis in the pathogenesis of human tuberculosis. Int J Tuberc Lung Dis. 2005; 9:375–83.38). Werle M, Schmal U, Hanna K, Kreuzer J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc Res. 2002; 56:284–92.
Article39). Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006; 98:1177–85.
Article40). Younce CW, Kolattukudy PE. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem J. 2010; 426:43–53.
Article41). Bidzhekov K, Zernecke A, Weber C. MCP-1 induces a novel transcription factor with proapoptotic activity. Circ Res. 2006; 98:1107–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress Responses and Apoptosis
- Endoplasmic Reticulum Stress and Diabetes
- Apoptotic Effect of Macrophages against Mycobacterium tuberculosis
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- Endoplasmic Reticulum Stress Activates Hepatic Macrophages through PERK-hnRNPA1 Signaling