Yonsei Med J.
2014 May;55(3):576-583. 10.3349/ymj.2014.55.3.576.
Expression of Glycolysis-Related Proteins in Solid Papillary Carcinoma of the Breast According to Basement Membrane Status
- Affiliations
-
- 1Department of Pathology, Ajou University College of Medicine, Suwon, Korea.
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. kjs1976@yuhs.ac
- KMID: 2068665
- DOI: http://doi.org/10.3349/ymj.2014.55.3.576
Abstract
- PURPOSE
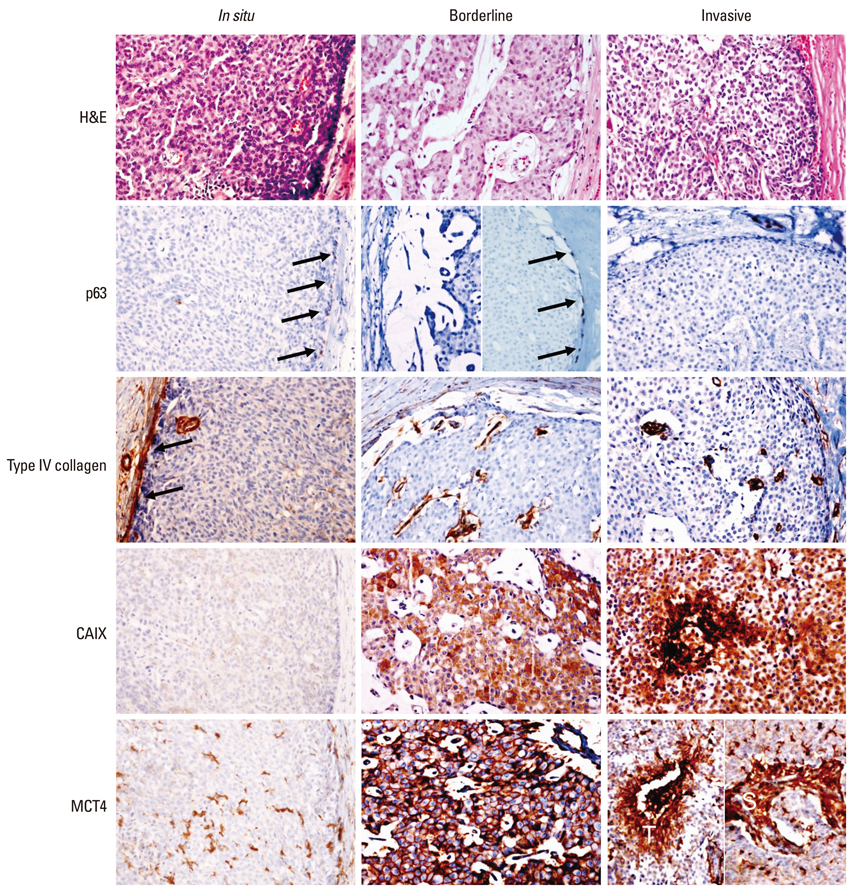
The aim of this study was to investigate the differences of expression in glycolysis-related proteins such as Glut-1, carbonic anhydrase (CA) IX, and monocarboxylate transporter (MCT) 4 according to the myoepithelial cell (MEC) and basement membrane (BM) status in solid papillary carcinoma (SPC) of the breast.
MATERIALS AND METHODS
Immunohistochemical evaluation of Glut-1, CAIX, and MCT4, as well as p63 and type IV collagen, were performed on 23 SPC cases.
RESULTS
Six and nine cases of SPC showed the presence and absence of myoepithelial cells, respectively, and eight cases belonged to the borderline status (p63-positive MEC on some areas of the outer tumor surface but not in others). BM was partially or completely absent in 14 cases and present in nine cases. SPC lacking BM more frequently showed high expression of CAIX than SPC with BM (p=0.037).
CONCLUSION
In SPC of the breast, a strong expression of CAIX seems to be associated with an increasing degree of loss of BM, which can be interpreted as BM degradation due to the induction of extracellular acidity with increasing expression of CAIX.
MeSH Terms
-
Adult
Aged
Basement Membrane/*metabolism
Breast Neoplasms/*metabolism
Carcinoma, Papillary/*metabolism
Excitatory Amino Acid Transporter 2/metabolism
Female
Glycolysis
Humans
Immunohistochemistry
Middle Aged
Monocarboxylic Acid Transporters/metabolism
Muscle Proteins/metabolism
Tumor Markers, Biological/*metabolism
Excitatory Amino Acid Transporter 2
Monocarboxylic Acid Transporters
Muscle Proteins
Tumor Markers, Biological
Figure
Reference
-
1. Maluf HM, Koerner FC. Solid papillary carcinoma of the breast. A form of intraductal carcinoma with endocrine differentiation frequently associated with mucinous carcinoma. Am J Surg Pathol. 1995; 19:1237–1244.2. Nassar H, Qureshi H, Adsay NV, Visscher D. Clinicopathologic analysis of solid papillary carcinoma of the breast and associated invasive carcinomas. Am J Surg Pathol. 2006; 30:501–507.
Article3. Dickersin GR, Maluf HM, Koerner FC. Solid papillary carcinoma of breast: an ultrastructural study. Ultrastruct Pathol. 1997; 21:153–161.
Article4. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004; 4:891–899.
Article5. Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995; 36:1625–1632.6. Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994; 9:2877–2888.7. Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999; 343 Pt 2:281–299.
Article8. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.
Article9. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.
Article10. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145.
Article11. Tsang WY, Chan JK. Endocrine ductal carcinoma in situ (E-DCIS) of the breast: a form of low-grade DCIS with distinctive clinicopathologic and biologic characteristics. Am J Surg Pathol. 1996; 20:921–943.12. Tramm T, Kim JY, Tavassoli FA. Diminished number or complete loss of myoepithelial cells associated with metaplastic and neoplastic apocrine lesions of the breast. Am J Surg Pathol. 2011; 35:202–211.
Article13. Tsubura A, Shikata N, Inui T, Morii S, Hatano T, Oikawa T, et al. Immunohistochemical localization of myoepithelial cells and basement membrane in normal, benign and malignant human breast lesions. Virchows Arch A Pathol Anat Histopathol. 1988; 413:133–139.
Article14. Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982; 42:4763–4770.15. Wetzels RH, Holland R, van Haelst UJ, Lane EB, Leigh IM, Ramaekers FC. Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol. 1989; 134:571–579.16. Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3'-kinase. Cancer Res. 2002; 62:4469–4477.17. Kopacek J, Barathova M, Dequiedt F, Sepelakova J, Kettmann R, Pastorek J, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta. 2005; 1729:41–49.
Article18. Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007; 26:299–310.
Article19. Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011; 10:1772–1783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine Needle Aspiration Cytology of Solid Papillary Carcinoma of the Breast: Report of a case associated with mucinous carcinoma
- c-Myc-Induced Long Non-Coding RNA Small Nucleolar RNA Host Gene 7 Regulates Glycolysis in Breast Cancer
- The Expression of Glut-1, CAIX, and MCT4 in Mucinous Carcinoma
- Expression of the nm23 and E-cadherin Proteins in Breast Carcinoma
- Composite Follicular Variant of Papillary Carcinoma and Mucoepidermoid Carcinoma of Thyroid Gland: A Case Report