The Neuroprotective Effect of Treatment of Valproic Acid in Acute Spinal Cord Injury
- Affiliations
-
- 1Department of Neurosurgery, School of Medicine, Kyungpook National University, Daegu, Korea. jksung@knu.ac.kr
- 2Department of Anatomy, School of Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2066909
- DOI: http://doi.org/10.3340/jkns.2012.51.4.191
Abstract
OBJECTIVE
Valproic acid (VPA), as known as histone deacetylase inhibitor, has neuroprotective effects. This study investigated the histological changes and functional recovery from spinal cord injury (SCI) associated with VPA treatment in a rat model.
METHODS
Locomotor function was assessed according to the Basso-Beattie-Bresnahan scale for 2 weeks in rats after receiving twice daily intraperitoneal injections of 200 mg/kg VPA or the equivalent volume of normal saline for 7 days following SCI. The injured spinal cord was then examined histologically, including quantification of cavitation.
RESULTS
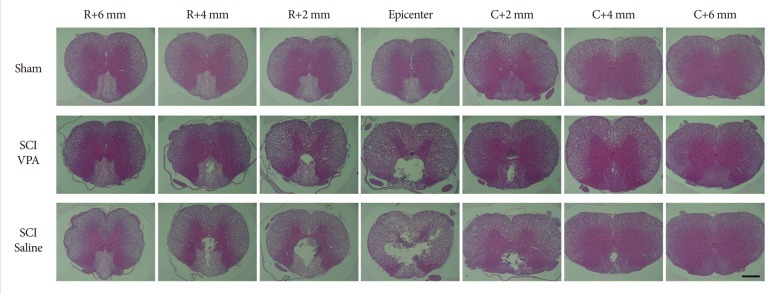
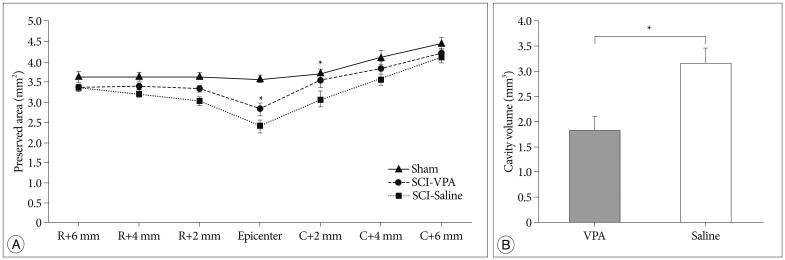
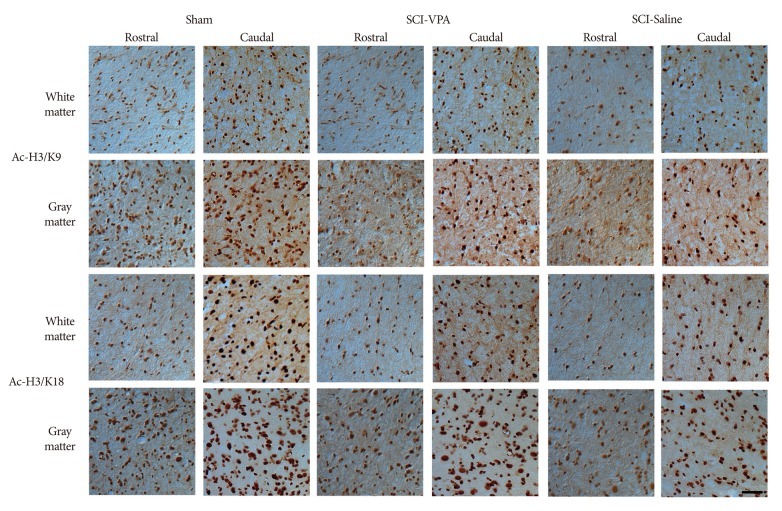
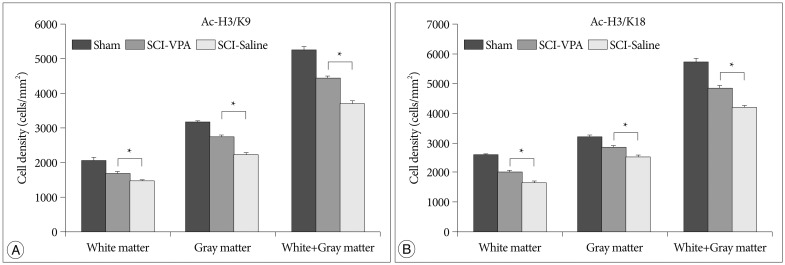
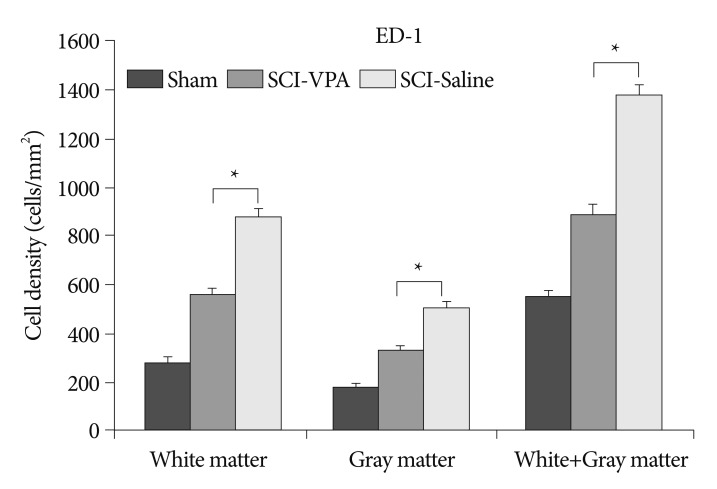
Basso-Beattie-Bresnahan scale scores in rats receiving VPA were significantly higher than in the saline group (p<0.05). The cavity volume in the VPA group was significantly reduced compared with the control (saline-injected) group (p<0.05). The level of histone acetylation recovered in the VPA group, while it was significantly decreased in the control rats (p<0.05). The macrophage level was significantly decreased in the VPA group (p<0.05).
CONCLUSION
VPA influences the restoration of hyperacetylation and reduction of the inflammatory reaction resulting from SCI, and is effective for histology and motor function recovery.
MeSH Terms
Figure
Cited by 4 articles
-
The Modulation of Neurotrophin and Epigenetic Regulators: Implication for Astrocyte Proliferation and Neuronal Cell Apoptosis After Spinal Cord Injury
Jong Heon Kim, Sung-Hoon Kim, Sung-Rae Cho, Ji Yong Lee, Ji Hyun Kim, Ahreum Baek, Hong Sun Jung
Ann Rehabil Med. 2016;40(4):559-567. doi: 10.5535/arm.2016.40.4.559.Epigenetic Regulation in the Brain after Spinal Cord Injury : A Comparative Study
Bit-Na-Ri Park, Seok Won Kim, Sung-Rae Cho, Ji Yong Lee, Young-Hee Lee, Sung-Hoon Kim
J Korean Neurosurg Soc. 2013;53(6):337-341. doi: 10.3340/jkns.2013.53.6.337.Valproic Acid Increases Expression of Neuronal Stem/Progenitor Cell in Spinal Cord Injury
Woo-Seok Bang, Kyoung-Tae Kim, Dae-Chul Cho, Hye-Jeong Kim, Joo-Kyung Sung
J Korean Neurosurg Soc. 2013;54(1):8-13. doi: 10.3340/jkns.2013.54.1.8.Effects of Tumor Necrosis Factor Alpha Blocker Adalimumab in Experimental Spinal Cord Injury
Alp Özgün Börcek, Soner Çivi, Özgür Öcal, Özlem Gülbahar
J Korean Neurosurg Soc. 2015;57(2):73-76. doi: 10.3340/jkns.2015.57.2.73.
Reference
-
1. Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978; 39:236–253. PMID: 713489.2. Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978; 39:254–266. PMID: 713490.3. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139:244–256. PMID: 8654527.
Article4. Beattie MS. Inflammation and apoptosis : linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004; 10:580–583. PMID: 15567326.
Article5. Blaheta RA, Nau H, Michaelis M, Cinatl J Jr. Valproate and valproate-analogues : potent tools to fight against cancer. Curr Med Chem. 2002; 9:1417–1433. PMID: 12173980.6. Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989; 91:15–34. PMID: 2746287.
Article7. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006; 5:769–784. PMID: 16955068.
Article8. Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984; 251:45–52. PMID: 6361287.
Article9. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997; 277:1597–1604. PMID: 9168289.
Article10. Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006; 59:970–975. PMID: 16607616.
Article11. Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002; 99:2258–2263. PMID: 11854521.
Article12. Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007; 149:203–212. PMID: 17850978.
Article13. Cho DC, Cheong JH, Yang MS, Hwang SJ, Kim JM, Kim CH. The effect of minocycline on motor neuron recovery and neuropathic pain in a rat model of spinal cord injury. J Korean Neurosurg Soc. 2011; 49:83–91. PMID: 21519495.
Article14. Cui SS, Yang CP, Bowen RC, Bai O, Li XM, Jiang W, Zhang X. Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats. Brain Res. 2003; 975:229–236. PMID: 12763612.
Article15. Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE. 2010; 5:e11383. PMID: 20614021.
Article16. Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006; 70:1876–1884. PMID: 16946032.
Article17. Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008; 155:567–572. PMID: 18640245.
Article18. Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001; 11:89–94. PMID: 11179877.
Article19. Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I, et al. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res. 2009; 65:35–43. PMID: 19463867.
Article20. Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004; 16:53–61. PMID: 14676570.
Article21. Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002; 99:9450–9455. PMID: 12082184.
Article22. Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001; 20:6969–6978. PMID: 11742974.
Article23. Hall ED, Springer JE. Neuroprotection and acute spinal cord injury : a reappraisal. NeuroRx. 2004; 1:80–100. PMID: 15717009.24. Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons : involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002; 80:589–597. PMID: 11841566.
Article25. Heneka MT, Gavrilyuk V, Landreth GE, O'Banion MK, Weinberg G, Feinstein DL. Noradrenergic depletion increases inflammatory responses in brain : effects on IkappaB and HSP70 expression. J Neurochem. 2003; 85:387–398. PMID: 12675915.
Article26. Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005; 17:664–671. PMID: 16226449.
Article27. Jeong MR, Hashimoto R, Senatorov VV, Fujimaki K, Ren M, Lee MS, et al. Valproic acid, a mood stabilizer and anticonvulsant, protects rat cerebral cortical neurons from spontaneous cell death : a role of histone deacetylase inhibition. FEBS Lett. 2003; 542:74–78. PMID: 12729901.
Article28. Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004; 4:336–344. PMID: 15289798.
Article29. Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008; 7:854–868. PMID: 18827828.
Article30. Khalatbary AR, Tiraihi T, Boroujeni MB, Ahmadvand H, Tavafi M, Tamjidipoor A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010; 1306:168–175. PMID: 19815005.
Article31. Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system : histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005; 4:41–50. PMID: 15723612.
Article32. Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003; 20:1017–1027. PMID: 14588118.
Article33. Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003; 23:4219–4227. PMID: 12764110.
Article34. Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005; 158:58–66. PMID: 15589038.
Article35. Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. J Surg Res. 2011; 166:280–289. PMID: 20018302.
Article36. Nalivaeva NN, Belyaev ND, Turner AJ. Sodium valproate : an old drug with new roles. Trends Pharmacol Sci. 2009; 30:509–514. PMID: 19762089.37. Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia : Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium. 2004; 36:303–311. PMID: 15261486.
Article38. Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000; 19:4310–4322. PMID: 10944114.
Article39. Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005; 79:340–350. PMID: 15605375.
Article40. Penas C, Verdú E, Asensio-Pinilla E, Guzmán-Lenis MS, Herrando-Grabulosa M, Navarro X, et al. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. 2011; 178:33–44. PMID: 21241777.
Article41. Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009; 106:12489–12493. PMID: 19666625.
Article42. Pérez M, Rojo AI, Wandosell F, Díaz-Nido J, Avila J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochem J. 2003; 372:129–136. PMID: 12578563.
Article43. Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001; 276:36734–36741. PMID: 11473107.
Article44. Rekling JC. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci Lett. 2003; 335:167–170. PMID: 12531459.
Article45. Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats : potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004; 89:1358–1367. PMID: 15189338.
Article46. Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004; 10:685–692. PMID: 15229516.
Article47. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies : promise on the horizon. Neurosurg Focus. 2008; 25:E2. PMID: 18980476.48. Shin YC, Choi KY, Kim WG. Cyclosporin A has a protective effect with induced upregulation of Hsp70 and nNOS on severe spinal cord ischemic injury in rabbits. J Invest Surg. 2007; 20:113–120. PMID: 17454396.
Article49. Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007; 26:464–472. PMID: 17398106.
Article50. Stepień K, Tomaszewski M, Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005; 57:561–569. PMID: 16227638.51. von Euler M, Seiger A, Sundström E. Clip compression injury in the spinal cord : a correlative study of neurological and morphological alterations. Exp Neurol. 1997; 145:502–510. PMID: 9217086.
Article52. Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia : the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011; 31:52–57. PMID: 20978517.
Article53. Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003; 126:1628–1637. PMID: 12805103.
Article54. Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008; 210:531–542. PMID: 18234195.
Article55. Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993; 11(Suppl 1):13–22. PMID: 8445198.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in the Pathophysiology and Treatment of Acute Spinal Cord Injury
- Combined Method of Neuronal Cell-Inducible Vector and Valproic Acid for Enhanced Gene Expression under Hypoxic Conditions
- Case of Decreased Serum Valproic Acid Concentration During Concomitant Use of Meropenem in Patients with Traumatic Brain Injury
- The Change of Total Ascorbic Acid Level in the Experimental Spinal Cord Injury
- Spinal Cord Injury and Related Clinical Trials