Obstet Gynecol Sci.
2015 Sep;58(5):333-339. 10.5468/ogs.2015.58.5.333.
Placental expression of D6 decoy receptor in preeclampsia
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. mjohmd@korea.ac.kr
- 2Department of Obstetrics and Gynecology, Bundang Cheil Women's Hospital, Seongnam, Korea.
- 3Department of Obstetrics and Gynecology, Kyunghee University College of Medicine, Seoul, Korea.
- KMID: 2058455
- DOI: http://doi.org/10.5468/ogs.2015.58.5.333
Abstract
OBJECTIVE
The purpose of this study was to investigate the expression of the D6 decoy receptor that can bind chemokines and target them for degradation, resulting in inhibition of inflammation in placentas from preeclamptic and normal pregnancies.
METHODS
The current study was carried out in 35 pregnant women (23 patients with preeclampsia and 12 healthy, normotensive pregnant women) during the third trimester of pregnancy. The expressions of D6 decoy receptor in the placenta were determined with real time reverse transcriptase polymerase chain reaction and western blotting.
RESULTS
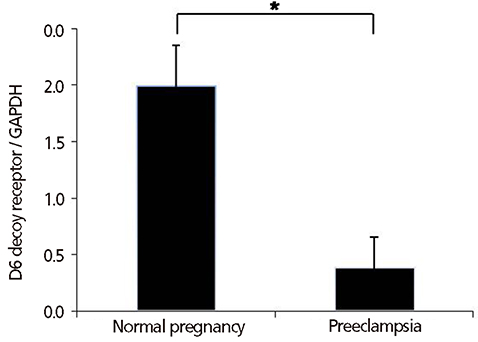
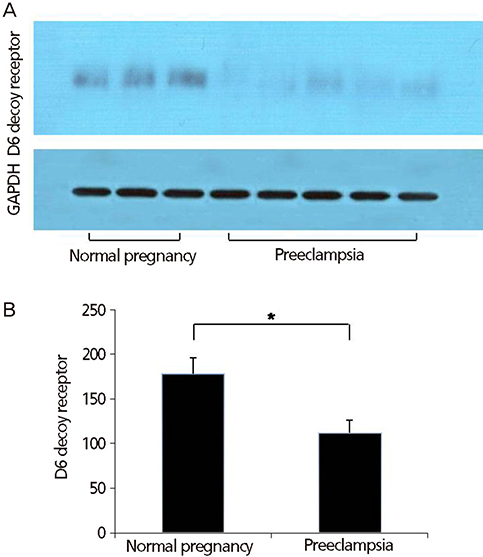
The mRNA and protein of D6 decoy receptor were detected in all of placentas from preeclamptic and normal pregnancies. Placental D6 decoy receptor mRNA expression was significantly lower in patients with preeclampsia than in patients with normal pregnancies. Western blot analyses revealed decreased protein expression in cases of preeclampsia.
CONCLUSION
The expression of the D6 decoy receptor in preeclamptic placentas was significantly lower than in normal placentas. Further studies are needed to clarify the underlying mechanisms that link decreased expression of placental D6 decoy receptor and preeclampsia.
Keyword
MeSH Terms
Figure
Reference
-
1. Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response: a review. Placenta. 2003; 24:Suppl A. S21–S27.2. Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2008; 140:21–26.3. Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006; 11:309–316.4. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999; 180(2 Pt 1):499–506.5. Nibbs RJ, McLean P, McCulloch C, Riboldi-Tunnicliffe A, Blair E, Zhu Y, et al. Structure-function dissection of D6, an atypical scavenger receptor. Methods Enzymol. 2009; 460:245–261.6. Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006; 6:907–918.7. Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, et al. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004; 172:4972–4976.8. Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001; 158:867–877.9. Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, et al. Protection against inflammation-and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007; 104:2319–2324.10. Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005; 35:1342–1346.11. Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999; 20:229–233.12. Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005; 73:237–243.13. Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009; 30:313–319.14. Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004; 89:6155–6167.15. Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998; 105:632–640.16. Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002; 186:158–166.17. Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007; 76:61–67.18. Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007; 178:5949–5956.19. Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006; 168:445–452.20. Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003; 101:227–231.21. Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007; 78:670–676.22. Rustveld LO, Kelsey SF, Sharma R. Association between maternal infections and preeclampsia: a systematic review of epidemiologic studies. Matern Child Health J. 2008; 12:223–242.23. Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006; 118:268–275.24. Beckmann I, Efraim SB, Vervoort M, Visser W, Wallenburg HC. Tumor necrosis factor-alpha in whole blood cultures of preeclamptic patients and healthy pregnant and nonpregnant women. Hypertens Pregnancy. 2004; 23:319–329.25. Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006; 70:83–91.26. Yarim GF, Karahan S, Nisbet C. Elevated plasma levels of interleukin 1 beta, tumour necrosis factor alpha and monocyte chemotactic protein 1 are associated with pregnancy toxaemia in ewes. Vet Res Commun. 2007; 31:565–573.27. Heikkila A, Tuomisto T, Hakkinen SK, Keski-Nisula L, Heinonen S, Yla-Herttuala S. Tumor suppressor and growth regulatory genes are overexpressed in severe early-onset preeclampsia: an array study on case-specific human preeclamptic placental tissue. Acta Obstet Gynecol Scand. 2005; 84:679–689.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Death Domain Receptor-3, Caspase-10, Insulin-Like Growth Factor Binding Protein-3 in the Placentas of Preeclampsia
- Placental Apoptosis in Preeclampsia
- Study on the Apoptosis and its Mediators in the Placentas of Preclampsia using cDNA Expression Array
- Maternal Preeclampsia and Bronchopulmonary Dysplasia
- Analysis of HLA-G expression in preeclampsia and intrauterine growth restriction