Int J Stem Cells.
2014 May;7(1):12-22.
Myelo-Enhancement by Astragalus Membranaceus in Male Albino Rats with Chemotherapy Myelo-Suppression. Histological and Immunohistochemical Study
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt. noha_afifi@windowslive.com
Abstract
- BACKGROUND AND OBJECTIVES
Myelo-suppression is the most common toxicity encountered in the oncology clinic today. This study was planned to investigate the possible protective and therapeutic role of the traditional Chinese Medicinal Herb; Astragalus Membranaceus (AM), on chemotherapy-induced myelosuppression.
METHODS AND RESULTS
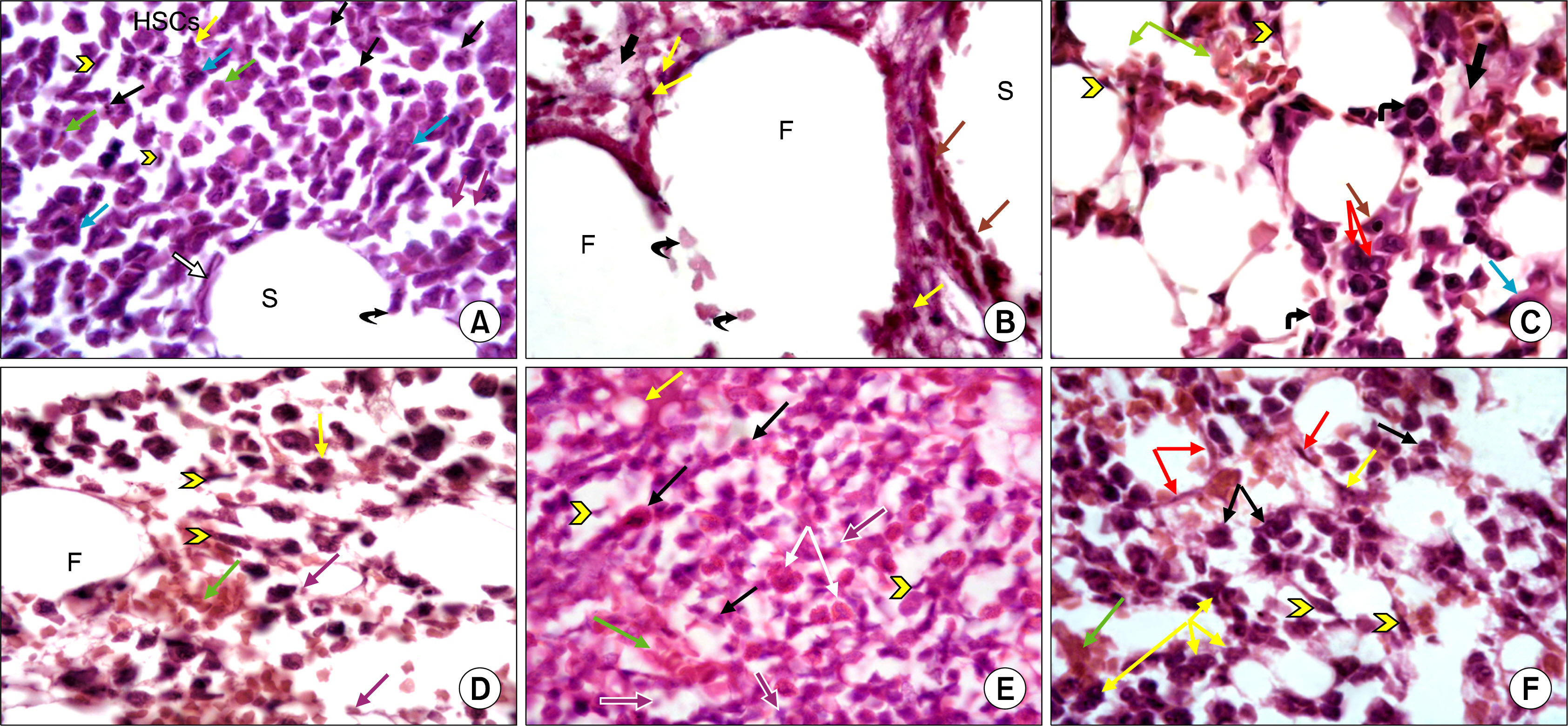
This study was carried out on thirty six adult male albino rats. They were divided into: Group I Control Group (n=6) received a vehicle of phosphate buffered saline (PBS) solution. Group II (n=12) were injected I.P. with cyclophosphamide (CY) for 3 days (gIIa n =6) and continued for one more week to receive AM orally (gIIb n=6). Group III (n=6) received CY I.P. together with AM orally for 3 days. Group IV (n=12) received AM orally for one week (gIVa n=6) and continued for extra three days receiving CY I.P. with AM orally (gIVb n=6). Blood samples were analysed for Total Leucocytic Count and Lymphocytic Count. Counting of CD34 +ve cells in bone marrow was performed by flowcytometry. Bone marrow sections were subjected to H&E stain as well as immunohistochemical staining for anti- CD20 antibody. The mean area % of cellular bone marrow regions occupied by developing haemopoietic cells, mean area of fat cells and mean number of CD20 immunopositive B lymphocytes in the bone marrow were measured by histomorphometric studies and statistically compared. AM proved to have a myelo-protective and myelo-therapeutic capacity, evidenced at both laboratory and morphological levels.
CONCLUSIONS
The greatest myelo-potentiating effect of AM was achieved when supplied before and together with CY therapy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006. 106:2258–2266.
Article2. Pass GJ, Carrie D, Boylan M, Lorimore S, Wright E, Houston B, Henderson CJ, Wolf CR. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005. 65:4211–4217.
Article3. Martín M, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, Rodríguez-Lescure A, Grosse R, Calvo L, Fernandez-Chacón C, Roset M, Antón A, Isla D, del Prado PM, Iglesias L, Zaluski J, Arcusa A, López-Vega JM, Muñoz M, Mel JR. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006. 17:1205–1212.
Article4. Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004. 70:261–264.
Article5. Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005. 10:427–437.
Article6. Wang J, Tong X, Li P, Cao H, Su W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J Ethnopharmacol. 2012. 139:788–795.
Article7. Li J, Zhong Y, Li H, Zhang N, Ma W, Cheng G, Liu F, Liu F, Xu J. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. Int J Biol Macromol. 2011. 49:362–368.
Article8. Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004. 320:1103–1111.
Article9. Macedo A, Orfão A, Vidriales MB, López-Berges MC, Valverde B, González M, Caballero MD, Ramos F, Martínez M, Fernández-Calvo J, et al. Characterization of aberrant phenotypes in acute myeloblastic leukemia. Ann Hematol. 1995. 70:189–194.
Article10. Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. London, New York & New Delhi: Arnold publisher;2001. 111–162.11. Bancroft JD, Cook HC. Immunocytochemistry. Manual of histological techniques and their diagnostic application. 2nd ed. Edinburgh, New York: Churchill Livingstone;1994. 263–325.12. Richard H. “Chapter 7: B Lymphocyte Development and Biology”. Paul W, editor. Fundamental Immunology. 6th ed. Philidelphia, PA: Lippincott Williams & Wilkins;2008. 237–269.13. Armitage P, Berry G. Armitage P, Berry G (Geoffrey), editors. Statistical methods in medical reseach. 3rd ed. London: Blackwell Scientific Publications;1994. 12–48.14. Haubitz M. Acute and Long-term Toxicity of Cyclophosphamide. Transplantationsmedizin. 2007. 19:26–31.15. Zhao AB, Yu B, Wu XL, Cao KJ, Li EQ, Li QM, Chen XY. Protective effects on myelosuppression mice treated by three different classic Chinese medicine formulae. Pharmacogn Mag. 2011. 7:133–140.
Article16. Underwood JCE. Cellular injury in Part 1 of General and Systemic pathology. fourth edition. ElsevierInc;2007. chapter 6. 105–107.17. Ross M, Pawlina W. Bone marrow and formation of blood cells (haemopoiesis). Histology: A Text and Atlas with Correlated Cell and Molecular Biology. 6th ed. Baltimore, Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins;2011. 289–300.18. Liu S, Hu P, Hou Y, Li P, Li X, Tian Q. The additive effect of mesenchymal stem cells and bone morphogenetic protein 2 on γ-irradiated bone marrow in mice. Cell Biochem Biophys. 2011. 61:539–550.
Article19. McGuire TR, Brusnahan SK, Bilek LD, Jackson JD, Kessinger MA, Berger AM, Garvin KL, O’Kane BJ, Tuljapurkar SR, Sharp JG. Inflammation associated with obesity: relationship with blood and bone marrow endothelial cells. Obesity (Silver Spring). 2011. 19:2130–2136.
Article20. Young B, Lowe J, Stevens A, Heath J. Blood. Wheater’s Functional Histology A Text and Colour Atlas. 5th ed. Philadelphia, USA: Churchill Livingstone Elsevier;2007. 42–61.21. Lv Y, Feng X, Zhu B. Study on effect of Astragalus membranaceus injection on hematopoiesis in anemic mice with myelosuppression. Zhong Yao Cai. 2005. 28:791–793.22. Zhu X, Zhu B. Effect of Astragalus membranaceus injection on megakaryocyte hematopoiesis in anemic mice. Hua Xi Yi Ke Da Xue Xue Bao. 2001. 32:590–592.23. Wei H, Sun R, Xiao W, Feng J, Zhen C, Xu X, Tian Z. Traditional Chinese medicine Astragalus reverses predominance of Th2 cytokines and their up-stream transcript factors in lung cancer patients. Oncol Rep. 2003. 10:1507–1512.
Article24. Chen Y, Zhu B, Zhang L, Yan S, Li J. Experimental study of the bone marrow protective effect of a traditional Chinese compound preparation. Phytother Res. 2009. 23:823–826.
Article25. Cho WC, Leung KN. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol. 2007. 113:132–141.
Article26. Syrjälä M1 Ruutu T, Jansson SE. A flow cytometric assay of CD34-positive cell populations in the bone marrow. Br J Haematol. 1994. 88:679–684.
Article27. Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004. 16:119–129.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Endophytic Diaporthe Species Isolated from the Leaves of Astragalus membranaceus in Korea
- Effect of Puffing in the Extraction of Active Ingredients from the Roots of Paeonia lactiflora and Astragalus membranaceus
- iNOS Induction by Polysaccharide Isolated from Astragalus membranaceus
- Complementary Therapies and Cancer Treatment
- The Effects of Astragalus Membranaceus on Repeated Restraint Stress-induced Biochemical and Behavioral Responses