Clin Exp Vaccine Res.
2014 Jan;3(1):91-99. 10.7774/cevr.2014.3.1.91.
Immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine: an open-labeled, randomized trial in healthy Korean children
- Affiliations
-
- 1Department of Pediatrics, Kyung Hee University Hospital, Kung Hee University School of Medicine, Seoul, Korea. sunghocha@khu.ac.kr
- 2Department of Pediatrics, Hangang Sacred Heart Hospital, Hallym University Medical Center, Hallym University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 4Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 5Department of Statistics, GlaxoSmithKline Biologicals, Wavre, Belgium.
- 6Department of Pediatrics, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 7Clinical Development, MMR/V Vaccines, Global Vaccines Development, GlaxoSmithKline Biologicals-Philadelphia, King of Prussia, PA, USA.
- KMID: 2049099
- DOI: http://doi.org/10.7774/cevr.2014.3.1.91
Abstract
- PURPOSE
This study (NCT00751348) evaluated the immunogenicity and safety of a combined measles-mumps-rubella-varicella (MMRV) vaccine compared to co-administration of measles-mumps-rubella and varicella (MMR+V) vaccines in Korean children during their second year of life.
MATERIALS AND METHODS
Healthy children aged 11-24 months received one dose of MMRV or MMR+V. Antibody titers against measles, mumps and rubella were measured using enzyme-linked immunosorbent assay and against varicella using an immunofluorescence assay. Parents/guardians recorded adverse events in diary cards for up to 43 days post-vaccination. The primary objective was to demonstrate non-inferiority of MMRV to MMR+V for all antigens in terms of seroconversion rates (SCRs), defined as a group difference with a lower limit of the 95% confidence interval (CI)>-10%.
RESULTS
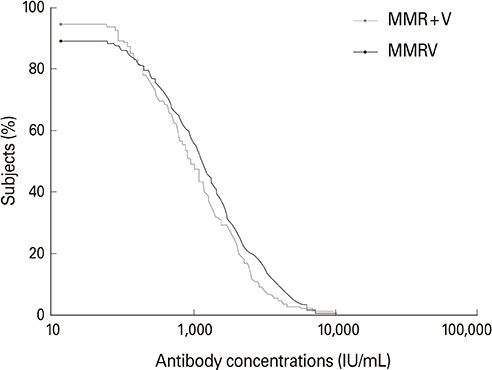
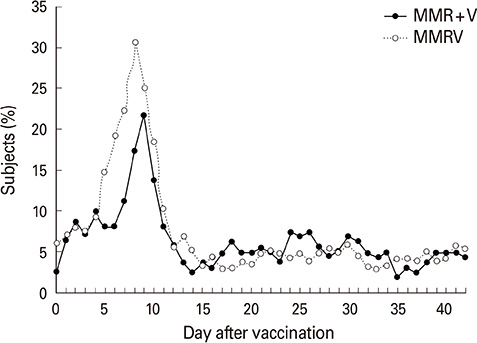
Of 474 subjects enrolled, 458 (MMRV, 301; MMR+V, 157) were included in the according-to-protocol cohort. For measles (98.0% vs. 99.4%), rubella (99.7% vs. 100%) and varicella (98.9% vs. 100%) SCRs, the lower limits of the 95% CIs for group differences were greater than -10%; however, for mumps SCRs (88.8% vs. 94.2%), it was -10.40%. The primary objective of non-inferiority in mumps SCRs was therefore not met, although the observed group difference in a post-hoc analysis of anti-mumps antibodies using a plaque reduction neutralization assay was 0.39% with a 95% CI lower limit of -4.03%. Adverse events occurred at comparable frequencies for both groups, except for more frequent fever in MMRV recipients.
CONCLUSION
Based on the pre-specified non-inferiority criterion, SCRs of the MMRV vaccine were non-inferior to that elicited by MMR+V vaccines for all antigens except mumps.
Keyword
MeSH Terms
Figure
Reference
-
1. Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009; 84:349–360.2. Mumps virus vaccines. Wkly Epidemiol Rec. 2007; 82:51–60.3. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011; 86:301–316.4. Atkinson W, Wolfe S, Hamborsky J. Epidemiology and prevention of vaccine-preventable diseases. The Pink Book: course textbook. 12th ed. Washington, DC: Public Health Foundation;2011.5. Lee H, Kim HW, Cho HK, Park EA, Choi KM, Kim KH. Reappraisal of MMR vaccines currently used in Korea. Pediatr Int. 2011; 53:374–380.
Article6. Centers for Disease Control and Prevention (CDC). Elimination of measles: South Korea, 2001-2006. MMWR Morb Mortal Wkly Rep. 2007; 56:304–307.7. Choe YJ, Lee ST, Song KM, Cho H, Bae GR, Lee JK. Surveillance and control of rubella in the Republic of Korea from 2001 to 2009: the necessity for enhanced surveillance to monitor congenital rubella syndrome. Osong Public Health Res Perspect. 2010; 1:23–28.
Article8. Choi WS, Noh JY, Huh JY, et al. Seroprevalence of varicella-zoster virus in Korea. J Med Virol. 2010; 82:2123–2126.
Article9. Czajka H, Schuster V, Zepp F, Esposito S, Douha M, Willems P. A combined measles, mumps, rubella and varicella vaccine (Priorix-Tetra): immunogenicity and safety profile. Vaccine. 2009; 27:6504–6511.
Article10. Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007; 56:1–40.11. Vesikari T, Sadzot-Delvaux C, Rentier B, Gershon A. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr Infect Dis J. 2007; 26:632–638.
Article12. Stück B, Stehr K, Bock HL. Concomitant administration of varicella vaccine with combined measles, mumps, and rubella vaccine in healthy children aged 12 to 24 months of age. Asian Pac J Allergy Immunol. 2002; 20:113–120.13. Nolan T, McIntyre P, Roberton D, Descamps D. Reactogenicity and immunogenicity of a live attenuated tetravalent measles-mumps-rubella-varicella (MMRV) vaccine. Vaccine. 2002; 21:281–289.
Article14. Goh P, Lim FS, Han HH, Willems P. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection. 2007; 35:326–333.
Article15. Rümke HC, Loch HP, Hoppenbrouwers K, et al. Immunogenicity and safety of a measles-mumps-rubella-varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine. 2011; 29:3842–3849.
Article16. Schuster V, Otto W, Maurer L, et al. Immunogenicity and safety assessments after one and two doses of a refrigerator-stable tetravalent measles-mumps-rubella-varicella vaccine in healthy children during the second year of life. Pediatr Infect Dis J. 2008; 27:724–730.
Article17. Vesikari T, Baer M, Willems P. Immunogenicity and safety of a second dose of measles-mumps-rubella-varicella vaccine in healthy children aged 5 to 6 years. Pediatr Infect Dis J. 2007; 26:153–158.
Article18. Knuf M, Habermehl P, Zepp F, et al. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr Infect Dis J. 2006; 25:12–18.
Article19. Mauldin J, Carbone K, Hsu H, Yolken R, Rubin S. Mumps virus-specific antibody titers from pre-vaccine era sera: comparison of the plaque reduction neutralization assay and enzyme immunoassays. J Clin Microbiol. 2005; 43:4847–4851.
Article20. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika. 1934; 26:404–413.
Article21. Knuf M, Faber J, Barth I, Habermehl P. A combination vaccine against measles, mumps, rubella and varicella. Drugs Today (Barc). 2008; 44:279–292.
Article22. Shinefield H, Black S, Digilio L, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005; 24:665–669.
Article23. Gillet Y, Steri GC, Behre U, et al. Immunogenicity and safety of measles-mumps-rubella-varicella (MMRV) vaccine followed by one dose of varicella vaccine in children aged 15 months-2 years or 2-6 years primed with measles-mumps-rubella (MMR) vaccine. Vaccine. 2009; 27:446–453.
Article24. Rentier B, Gershon AA, European Working. Consensus: varicella vaccination of healthy children: a challenge for Europe. Pediatr Infect Dis J. 2004; 23:379–389.25. Marcy SM. Pediatric combination vaccines: their impact on patients, providers, managed care organizations, and manufacturers. Am J Manag Care. 2003; 9:314–320.26. Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule. Am J Manag Care. 2003; 9:1 Suppl. S6–S12.27. Jacobsen SJ, Ackerson BK, Sy LS, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009; 27:4656–4661.
Article28. Klein NP, Fireman B, Yih WK, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010; 126:e1–e8.
Article29. Committee on Infectious Diseases. Policy statement: prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. Pediatrics. 2011; 128:630–632.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study of immunogenicity of measles, mumps and rubella vaccine prepared from human diploid cell
- Antibody Response and Adverse Reaction Following Immunization with MMR Vaccine Produced on Human Diploid Cells in Korean Children
- Update in varicella vaccination
- Seroprevalence of measles, mumps, rubella, and varicella-zoster antibodies in new female nurses in the Republic of Korea
- Modified Measles in an Anti-Measles Immunoglobulin G-negative Healthcare Worker who had Received Two Doses of Measles-Containing Vaccine