Clin Exp Vaccine Res.
2014 Jan;3(1):5-11. 10.7774/cevr.2014.3.1.5.
Inflammasomes in antiviral immunity: clues for influenza vaccine development
- Affiliations
-
- 1Division of Viral Infection, Department of Infectious Disease Control, International Research Center for Infectious Diseases, Institute of Medical Science, The University of Tokyo, Tokyo, Japan. ichinohe@ims.u-tokyo.ac.jp
- KMID: 2049091
- DOI: http://doi.org/10.7774/cevr.2014.3.1.5
Abstract
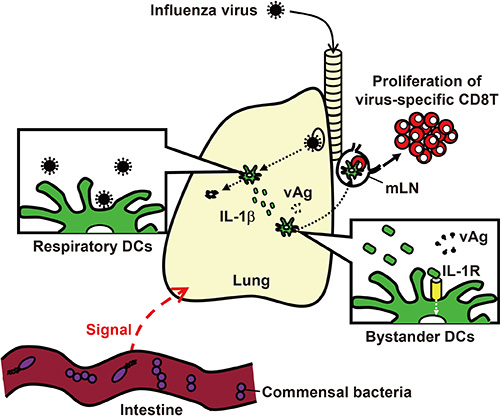
- Inflammasomes are cytosolic multiprotein complexes that sense microbial motifs or cellular stress and stimulate caspase-1-dependent cytokine secretion and cell death. Recently, it has become increasingly evident that both DNA and RNA viruses activate inflammasomes, which control innate and adaptive immune responses against viral infections. In addition, recent studies suggest that certain microbiota induce inflammasomes-dependent adaptive immunity against influenza virus infections. Here, we review recent advances in research into the role of inflammasomes in antiviral immunity.
MeSH Terms
Figure
Reference
-
1. Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013; 368:1888–1897.
Article2. Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009; 459:931–939.
Article3. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010; 327:291–295.
Article4. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384.
Article5. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004; 303:1529–1531.
Article6. Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004; 101:5598–5603.
Article7. Kato H, Sato S, Yoneyama M, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005; 23:19–28.
Article8. Rehwinkel J, Tan CP, Goubau D, et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010; 140:397–408.
Article9. Bracci L, Canini I, Puzelli S, et al. Type I IFN is a powerful mucosal adjuvant for a selective intranasal vaccination against influenza virus in mice and affects antigen capture at mucosal level. Vaccine. 2005; 23:2994–3004.
Article10. Ichinohe T, Iwasaki A, Hasegawa H. Innate sensors of influenza virus: clues to developing better intranasal vaccines. Expert Rev Vaccines. 2008; 7:1435–1445.
Article11. Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003; 21:3212–3218.
Article12. Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001; 75:5141–5150.
Article13. Koyama S, Aoshi T, Tanimoto T, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med. 2010; 2:25ra24.
Article14. Ichinohe T, Watanabe I, Ito S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005; 79:2910–2919.
Article15. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009; 27:229–265.
Article16. Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010; 10:210–215.
Article17. Bauernfeind F, Ablasser A, Bartok E, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011; 68:765–783.
Article18. Poeck H, Bscheider M, Gross O, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010; 11:63–69.
Article19. Burckstummer T, Baumann C, Bluml S, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009; 10:266–272.
Article20. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009; 458:509–513.
Article21. Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009; 458:514–518.
Article22. Roberts TL, Idris A, Dunn JA, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009; 323:1057–1060.
Article23. Rathinam VA, Jiang Z, Waggoner SN, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010; 11:395–402.
Article24. Ansari MA, Singh VV, Dutta S, et al. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol. 2013; 87:8606–8623.
Article25. Kerur N, Veettil MV, Sharma-Walia N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011; 9:363–375.
Article26. Johnson KE, Chikoti L, Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol. 2013; 87:5005–5018.
Article27. Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010; 11:404–410.
Article28. Ito M, Yanagi Y, Ichinohe T. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog. 2012; 8:e1002857.
Article29. Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol. 2013; 49:923–934.
Article30. Carter SD, Dent KC, Atkins E, et al. Direct visualization of the small hydrophobic protein of human respiratory syncytial virus reveals the structural basis for membrane permeability. FEBS Lett. 2010; 584:2786–2790.
Article31. Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax. 2013; 68:66–75.
Article32. Mitoma H, Hanabuchi S, Kim T, et al. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity. 2013; 39:123–135.
Article33. Netea MG, Nold-Petry CA, Nold MF, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009; 113:2324–2335.
Article34. Wang K, Xie S, Sun B. Viral proteins function as ion channels. Biochim Biophys Acta. 2011; 1808:510–515.
Article35. Pang IK, Iwasaki A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol Rev. 2012; 245:209–226.36. Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009; 30:556–565.
Article37. Thomas PG, Dash P, Aldridge JR Jr, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009; 30:566–575.
Article38. Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009; 206:79–87.
Article39. Wang L, Manji GA, Grenier JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002; 277:29874–29880.
Article40. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426.41. Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol. 2013; 14:246–253.
Article42. Fernandez-Sesma A, Marukian S, Ebersole BJ, et al. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006; 80:6295–6304.
Article43. Smed-Sorensen A, Chalouni C, Chatterjee B, et al. Influenza A virus infection of human primary dendritic cells impairs their ability to cross-present antigen to CD8 T cells. PLoS Pathog. 2012; 8:e1002572.
Article44. Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000; 1:127–131.
Article45. Liniger M, Summerfield A, Ruggli N. MDA5 can be exploited as efficacious genetic adjuvant for DNA vaccination against lethal H5N1 influenza virus infection in chickens. PLoS One. 2012; 7:e49952.
Article46. McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase: increased expression with reduced muscle damage. Gene Ther. 2001; 8:1264–1270.
Article47. Sasaki S, Amara RR, Oran AE, Smith JM, Robinson HL. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat Biotechnol. 2001; 19:543–547.
Article48. Okuda K, Kawamoto S, Fukushima J. Cytokine and costimulatory factor-encoding plasmids as adjuvants for DNA vaccination. Methods Mol Med. 2000; 29:197–204.
Article49. Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004; 428:561–564.
Article50. Chen Z, Yoshikawa T, Kadowaki S, et al. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J Gen Virol. 1999; 80(Pt 10):2559–2564.
Article51. Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc Natl Acad Sci U S A. 2010; 107:4281–4286.
Article52. Luckay A, Sidhu MK, Kjeken R, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007; 81:5257–5269.
Article53. Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA. Delivery of DNA vaccines using electroporation. Methods Mol Med. 2006; 127:73–82.
Article54. Khurana S, Wu J, Dimitrova M, et al. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis. 2013; 208:413–417.
Article55. Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012; 481:81–84.
Article56. Yamazaki T, Nagashima M, Ninomiya D, et al. Passive immune-prophylaxis against influenza virus infection by the expression of neutralizing anti-hemagglutinin monoclonal antibodies from plasmids. Jpn J Infect Dis. 2011; 64:40–49.57. Balazs AB, Bloom JD, Hong CM, Rao DS, Baltimore D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat Biotechnol. 2013; 31:647–652.
Article58. Limberis MP, Adam VS, Wong G, et al. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci Transl Med. 2013; 5:187ra72.
Article59. Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140:821–832.
Article60. Mann CJ, Anguela XM, Montane J, et al. Molecular signature of the immune and tissue response to non-coding plasmid DNA in skeletal muscle after electrotransfer. Gene Ther. 2012; 19:1177–1186.
Article61. Suschak J, Wang S, Remington K, Fitzgerald K, Lu S. Involvement of the Aim2 inflammasome pathway in generating antibody responses elicited by DNA vaccination. J Immunol. 2013; 190:123.20.62. Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013; 13:321–335.
Article63. Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011; 108:5354–5359.
Article64. Abt MC, Osborne LC, Monticelli LA, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012; 37:158–170.
Article65. Ganal SC, Sanos SL, Kallfass C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012; 37:171–186.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seasonal influenza and vaccine herd effect
- Influenza Vaccine
- Challenges in the development of T-cell–based universal influenza vaccines
- Cross-Protective Immune Responses Elicited by Live Attenuated Influenza Vaccines
- A Case of Severe Influenza Infection in a Child with Nephrotic Syndrome on Steroid Therapy