Ann Surg Treat Res.
2015 Jul;89(1):9-16. 10.4174/astr.2015.89.1.9.
CD44 expression in patients with combined hepatocellular cholangiocarcinoma
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 2Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 3Department of Surgery, Kangwon National University Hospital, Chuncheon, Korea. cgb3377@kangwon.ac.kr
- KMID: 2047708
- DOI: http://doi.org/10.4174/astr.2015.89.1.9
Abstract
- PURPOSE
Combined hepatocellular cholangiocarcinoma (ChC) is a rare type of primary liver cancer, which is thought to have a poorer prognosis than hepatocellular carcinoma (HCC). Cancer stem cells are associated with tumorigenesis, tumor progression, recurrence, metastasis, and poor prognosis in several malignancies including HCC. The aim of this study was to investigate the expression pattern of cancer stem cell markers in ChC and HCC, and to evaluate whether this pattern correlated to patient prognosis.
METHODS
Thirteen patients who underwent curative hepatic resection for ChC and 13 patients who underwent curative hepatic resection for HCC (matched control cases) were included. Immunohistochemical staining for cancer stem cell markers (cytokeratin [CK]7, CK19, C-kit, cluster of differentiation [CD] 44, CD133, and epithelial cell adhesion molecule) was performed and clinical outcomes were analyzed retrospectively.
RESULTS
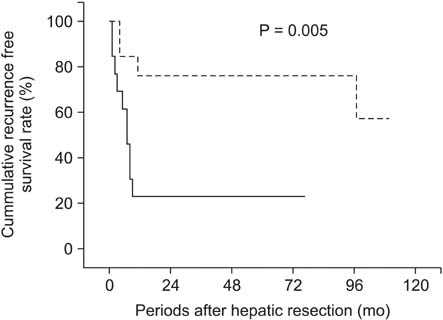
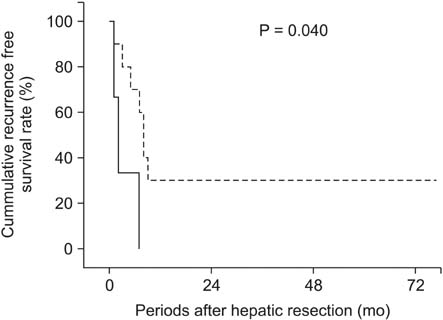
There was no significant difference in cancer stem cell marker expression between ChC and HCC. In ChC, the group that expressed CD44 showed earlier recurrence than the group that did not express CD44 (P = 0.040).
CONCLUSION
The expression of cancer stem cell markers in ChC did not show a different pattern compared to that found in HCC. The expression of cancer stem cell marker CD44 was associated with poor prognosis in patients with ChC.
Keyword
MeSH Terms
Figure
Reference
-
1. Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996; 11:758–764.2. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003; 33:283–287.3. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005; 189:120–125.4. Portolani N, Baiocchi GL, Coniglio A, Piardi T, Grazioli L, Benetti A, et al. Intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma: a Western experience. Ann Surg Oncol. 2008; 15:1880–1890.5. Yu XH, Xu LB, Zeng H, Zhang R, Wang J, Liu C. Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2011; 10:620–625.6. Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol. 2012; 19:1628–1636.7. Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011; 45:69–75.8. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002; 94:2040–2046.9. Lo RC, Ng IO. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer. 2013; 2:84–92.10. Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008; 47:1544–1556.11. Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, et al. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010; 16:4688–4694.12. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013; 123:1911–1918.13. Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006; 49:138–151.14. Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010; 59:953–962.15. Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012; 56:1804–1816.16. Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008; 27:1749–1758.17. Song W, Li H, Tao K, Li R, Song Z, Zhao Q, et al. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008; 62:1212–1218.18. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC Press;2010.19. Endo K, Terada T. Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, p53 expression, and patient survival. J Hepatol. 2000; 32:78–84.20. Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, et al. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol. 2013; 140:329–340.21. Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013; 37:496–505.22. Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of 'subtypes with stem-cell feature' in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015; 35:1024–1035.23. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990; 61:1303–1313.24. Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011; 11:254–267.25. Henry JC, Park JK, Jiang J, Kim JH, Nagorney DM, Roberts LR, et al. miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2010; 403:120–125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Study for CD44v6 in Hepatocellular Carcinoma and Cholangiocarcinoma
- Combined Hepatocellular-Cholangiocarcinoma: Recent Progress in Pathology and Classification
- Combined Hepatocellular-cholangiocarcinoma
- Combined Hepatocellular-cholangiocarcinoma
- Immunohistochemieal Study of Expression of nm23 and CD44 Protein in