Lab Med Online.
2015 Oct;5(4):204-210. 10.3343/lmo.2015.5.4.204.
Evaluation of a Test Index Obtained by Coupling Protein with Specific Gravity of Screening Urine Test
- Affiliations
-
- 1Department of Laboratory Medicine, Hanyang University Medical Center, Seoul, Korea. ikpark@hanyang.ac.kr
- KMID: 2046382
- DOI: http://doi.org/10.3343/lmo.2015.5.4.204
Abstract
- BACKGROUND
The 24-hr urine protein excretion test is regarded as a standard for quantitative urinary protein analysis; however, collection of urine over 24 hr is troublesome and errors may occur during the process. We obtained a test index reflecting diluted or concentrated urine by coupling the results of a simple and rapid routine urine analysis and evaluated its usefulness as a marker that quantitatively reflects the 24-hr urine protein excretion.
METHODS
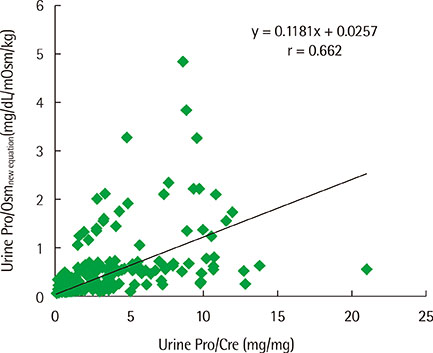
The estimated urine protein-osmolality ratio (Pro/Osm) was obtained by two linear regression equations between urine dipstick protein and natural logarithm of the protein concentration, and between urine specific gravity (SG) and urine osmolality (Osm). Sensitivity and specificity of 'estimated urine Pro/Osm' and the widely used urine protein-creatinine ratio were evaluated for their efficiency in diagnosing pathological proteinuria and nephrotic proteinuria based on 24-hr urine protein excretion.
RESULTS
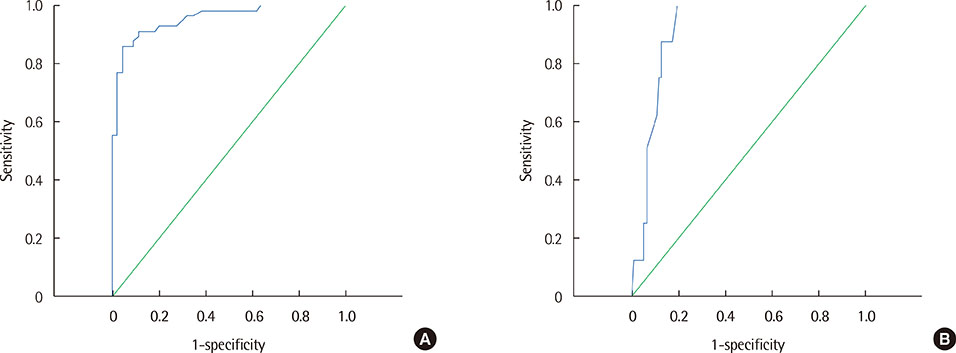
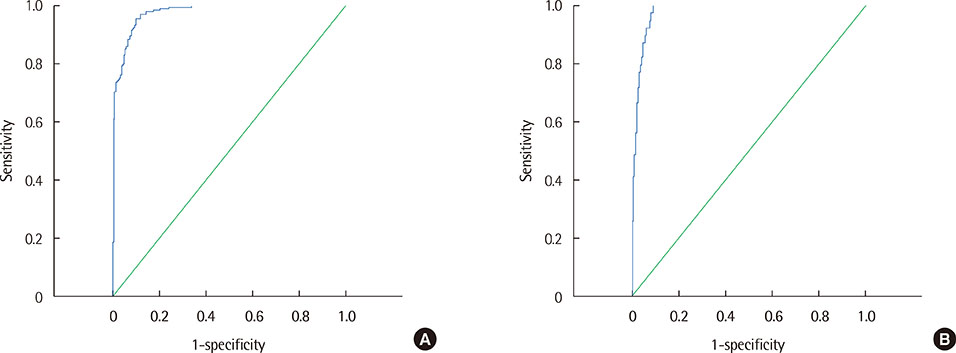
Moderate correlations were noted between protein concentration determined by the urine dipstick protein assay and natural logarithm of protein concentration (r=0.86) and between urine SG and urine Osm (r=0.74). The receiver operating characteristics analysis showed that an estimated urine Pro/Osm value of 0.045 had a sensitivity of 91.1% and a specificity of 88.6% for diagnosing pathological proteinuria, and a value of 0.204 had a sensitivity of 100% and a specificity of 80.4% for diagnosing nephrotic proteinuria.
CONCLUSIONS
Coupling the results of urine dipstick protein and urine SG determined by the routine analysis will provide additional useful information that will make the screening of renal diseases more cost-effective.
MeSH Terms
Figure
Reference
-
1. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010; 303:423–429.
Article2. McIntyre NJ, Taal MW. How to measure proteinuria? Curr Opin Nephrol Hypertens. 2008; 17:600–603.
Article3. Chotayaporn T, Kasitanon N, Sukitawut W, Louthrenoo W. Comparison of proteinuria determination by urine dipstick, spot urine protein creatinine index, and urine protein 24 hours in lupus patients. J Clin Rheumatol. 2011; 17:124–129.
Article4. Leung YY, Szeto CC, Tam LS, Lam CW, Li EK, Wong KC, et al. Urine protein-to-creatinine ratio in an untimed urine collection is a reliable measure of proteinuria in lupus nephritis. Rheumatology. 2007; 46:649–652.
Article5. Montero N, Soler MJ, Pascual MJ, Barrios C, Márquez E, Rodríguez E, et al. Correlation between the protein/creatinine ratio in spot urine and 24-hour urine protein. Nefrologia. 2012; 32:494–501.6. Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005; 51:1577–1586.
Article7. Serdaroglu E, Mir S. Protein-osmolality ratio for quantification of proteinuria in children. Clin Exp Nephrol. 2008; 12:354–357.
Article8. White SL, Yu R, Craig JC, Polkinghorne KR, Atkins RC, Chadban SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011; 58:19–28.
Article9. Pugia MJ, Wallace JF, Lott JA, Sommer R, Luke KE, Shihabi ZK, et al. Albuminuria and proteinuria in hospitalized patients as measured by quantitative and dipstick methods. J Clin Lab Anal. 2001; 15:295–300.
Article10. Imran S, Eva G, Christopher S, Flynn E, Henner D. Is specific gravity a good estimate of urine osmolality? J Clin Lab Anal. 2010; 24:426–430.
Article11. Antunes VV, Veronese FJ, Morales JV. Diagnostic accuracy of the protein/creatinine ratio in urine samples to estimate 24-h proteinuria in patients with primary glomerulopathies: a longitudinal study. Nephrol Dial Transplant. 2008; 23:2242–2246.
Article12. Park EY, Kim TY. How to interpret the protein/creatinine ratio in patients with low GFR. Nephrol Dial Transplant. 2009; 24:3892–3893.
Article13. Wilson DM, Anderson RL. Protein-osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. Am J Clin Pathol. 1993; 100:419–424.
Article14. Morgenstern BZ, Butani L, Woilan P, Wilson DM, Larson TS. Validity of protein-osmolality versus protein-creatinine ratios in the estimation of quantitative proteinuria from random samples of urine in children. Am J Kidney Dis. 2003; 41:760–766.
Article15. Kim HS, Cheon HW, Choe JH, Yoo KH, Hong YS, Lee JW, et al. Quantification of proteinuria in children using the urinary protein-osmolality ratio. Pediatr Nephrol. 2001; 16:73–76.
Article16. Newman DJ, Pugia MJ, Lott JA, Wallace JF, Hiar AM. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta. 2000; 294:139–155.
Article17. Makihara N, Yamasaki M, Morita H, Yamada H. A dipstick test combined with urine specific gravity improved the accuracy of proteinuria determination in pregnancy screening. Kobe J Med Sci. 2011; 56:E165–E172.18. Gai M, Motta D, Giunti S, Fop F, Masini S, Mezza E, et al. Comparison between 24-h proteinuria, urinary protein/creatinine ratio and dipstick test in patients with nephropathy: patterns of proteinuria in dipstick-negative patients. Scand J Clin Lab Invest. 2006; 66:299–307.
Article19. Ralston SH, Caine N, Richards I, O'Reilly D, Sturrock RD, Capell HA. Screening for proteinuria in a rheumatology clinic: comparison of dipstick testing, 24 hour urine quantitative protein, and protein/creatinine ratio in random urine samples. Ann Rheum Dis. 1988; 47:759–763.
Article20. Chadha V, Garg U, Alon US. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol. 2001; 16:374–382.
Article21. Constantiner M, Sehgal AR, Humbert L, Constantiner D, Arce L, Sedor JR, et al. A dipstick protein and specific gravity algorithm accurately predicts pathological proteinuria. Am J Kidney Dis. 2005; 45:833–841.
Article22. Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001; 74:63–67.
Article23. Torng S, Rigatto C, Rush DN, Nickerson P, Jeffery JR. The urine protein to creatinine ratio (P/C) as a predictor of 24-hour urine protein excretion in renal transplant patients. Transplantation. 2001; 72:1453–1456.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Annual Report of the Korean Association of External Quality Assessment Service on Urinalysis and Fecal Occult Blood Testing (2021)
- Performance Evaluation of the CLINITEK Novus Automated Urine Chemistry Analyzer
- Annual Report on the External Quality Assessment Scheme for Urinalysis and Faecal Occult Blood Testing in Korea (2015)
- Annual Report of the Korean Association of External Quality Assessment Service on Urinalysis and Fecal Occult Blood Testing (2018)
- Urinalysis: The Usefulness and Limitations of Urine Dipstick Testing