J Korean Orthop Assoc.
2015 Aug;50(4):320-327. 10.4055/jkoa.2015.50.4.320.
Preliminary Examination for Apoptosis and Cell Proliferation Related to Aging Process in the Anterior Cruciate Ligament Cells of the Human Degenerative Arthritic Knee Joint
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. hslee1003@catholic.ac.kr
- KMID: 2035869
- DOI: http://doi.org/10.4055/jkoa.2015.50.4.320
Abstract
- PURPOSE
We investigated the association between aging-induced apoptosis and cell proliferation in the anterior cruciate ligament (ACL) of aged patients.
MATERIALS AND METHODS
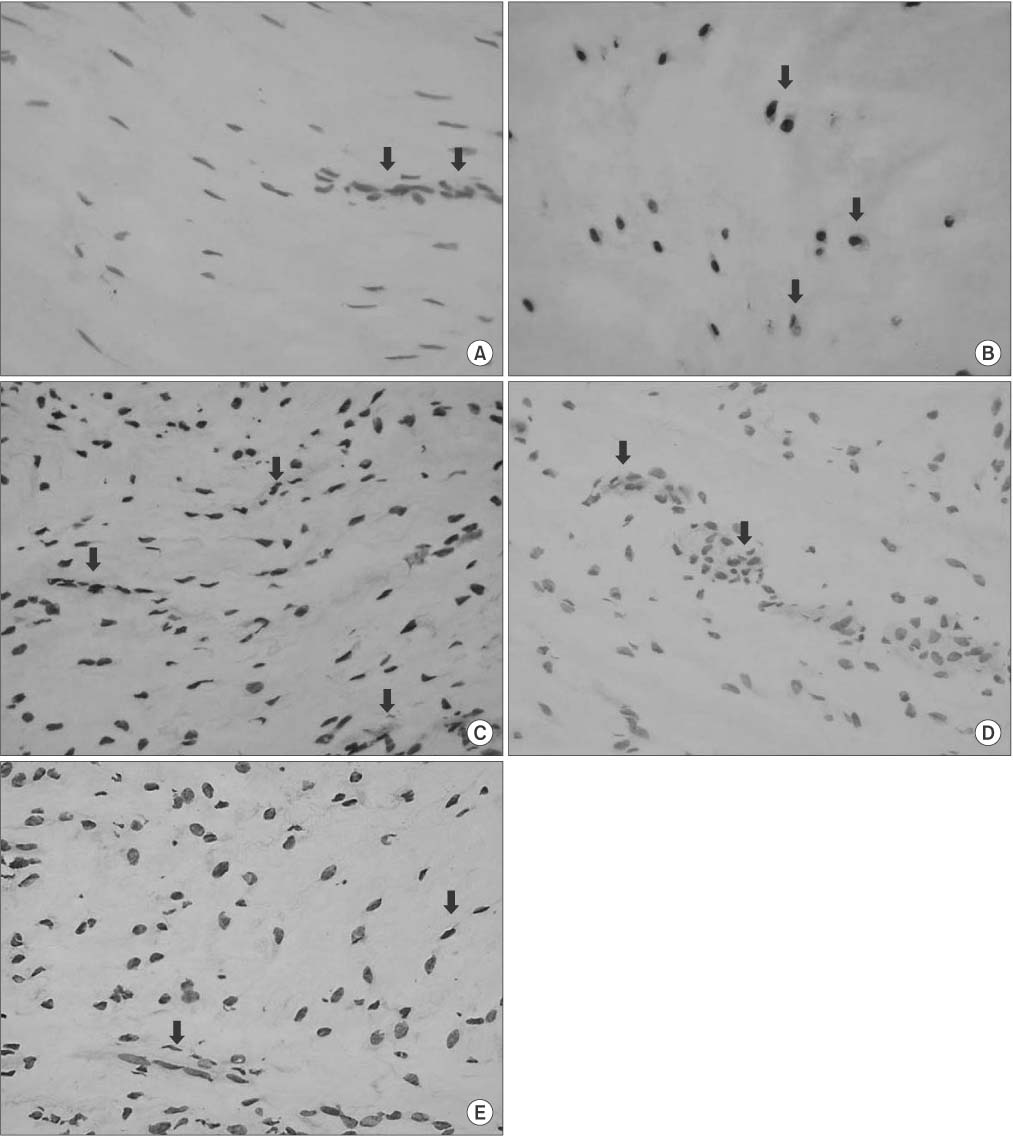
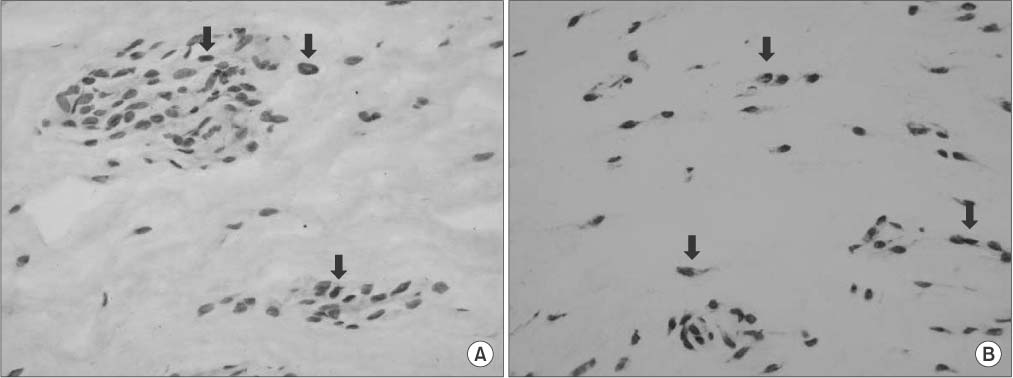
Twenty patients with osteoarthritis who underwent total knee replacement arthroplasty were enrolled in the study. They were divided into three groups according to age: group A (< or =65 years), group B (66-75 years), and group C (> or =76 years). The ACL tissue was obtained intraoperatively and subjected to terminal deoxynucleotidyl transferase dUTP nick-end labeling staining and immunohistochemistry to quantify the apoptosis and cell proliferation indices.
RESULTS
Apoptosis occurred in all groups, with the highest apoptosis index found in group C, followed by that in groups B and A. A statistically significant positive linear correlation was observed, with a 1-year increase in age resulting in an average increase of 1.49 in the apoptotic index. The lowest cell proliferation index was observed in group C, followed by that in group B and group A, with a 1-year increase in age resulting in an average decrease of 1.0 in the cell proliferation index, which was a statistically significant negative linear correlation. Consequently, a statistically significant negative correlation was confirmed between the apoptosis and cell proliferation indices, whereby an increase of 1.0 in the apoptosis index was concurrent with a decrease of 0.45 in the cell proliferation index.
CONCLUSION
Apoptosis occurred in the ACL of human knee joint. With increasing age, apoptosis increased and cell proliferation decreased.
MeSH Terms
Figure
Reference
-
1. Adams CS, Horton WE Jr. Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998; 250:418–425.
Article2. Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001; 44:1304–1312.3. Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998; 41:1632–1638.
Article4. Uysal M, Akpinar S, Bolat F, Cekin N, Cinar M, Cesur N. Apoptosis in the traumatic and degenerative tears of human meniscus. Knee Surg Sports Traumatol Arthrosc. 2008; 16:666–669.
Article5. Yoon HK, Seo SS, Choi JS, Park JK, Kim YJ. Apoptosis in the meniscus of human osteoarthritic knee. J Korean Orthop Res Soc. 2002; 5:43–54.6. Gyger O, Botteron C, Doherr M, Zurbriggen A, Schawalder P, Spreng D. Detection and distribution of apoptotic cell death in normal and diseased canine cranial cruciate ligaments. Vet J. 2007; 174:371–377.
Article7. Murakami H, Shinomiya N, Kikuchi T, Yoshihara Y, Nemoto K. Upregulated expression of inducible nitric oxide synthase plays a key role in early apoptosis after anterior cruciate ligament injury. J Orthop Res. 2006; 24:1521–1534.
Article8. Hasegawa A, Otsuki S, Pauli C, et al. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012; 64:696–704.
Article9. Krammer PH. CD95's deadly mission in the immune system. Nature. 2000; 407:789–795.
Article10. Waldherr K, Zurbriggen A, Spreng DE, Forterre S. In vitro cytoprotective effects of acetylsalicylic acid, carprofen, meloxicam, or robenacoxib against apoptosis induced by sodium nitroprusside in canine cruciate ligament cells. Am J Vet Res. 2012; 73:1752–1758.
Article11. Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995; 146:75–85.12. Hashimoto S, Setareh M, Ochs RL, Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997; 40:1749–1755.
Article13. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992; 119:493–501.
Article14. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182:311–322.
Article15. Bonfil RD, Gonzalez AD, Siguelboim D, et al. Immunohistochemical analysis of Ki-67, p21waf1/cip1 and apoptosis in marker lesions from patients with superficial bladder tumours treated with vinorelbine intravesical therapy in a preliminary phase I trial. BJU Int. 2001; 88:425–431.
Article16. Kimura T, Tanaka S, Haruma K, et al. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int J Oncol. 2000; 16:55–64.
Article17. Michael-Robinson JM, Reid LE, Purdie DM, et al. Proliferation, apoptosis, and survival in high-level microsatellite instability sporadic colorectal cancer. Clin Cancer Res. 2001; 7:2347–2356.18. Kim KW, Kim YS, Ha KY, et al. An autocrine or paracrine Fas-mediated counterattack: a potential mechanism for apoptosis of notochordal cells in intact rat nucleus pulposus. Spine (Phila Pa 1976). 2005; 30:1247–1251.19. Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998; 17:1675–1687.
Article20. Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999; 274:22532–22538.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Evolution of Cruciate Ligament Surgery of the Knee
- The Reconstruction of Anterior Cruciate Ligament Using Patellar Tendon under Arthroscopy
- Intraarticular Ganglion Arising from the Posterior Cruciate Ligament
- Arthroscopic repair and augumentation in acute anterior cruciate ligament injury: Surgical technique and preliminary results
- Anterior Cruciate Ligament Reconstruction: The Long Road from Science to Clinical Relevance