Korean Circ J.
2009 Oct;39(10):399-407. 10.4070/kcj.2009.39.10.399.
Progression and Observational Frequency of Atheromatous Plaques in Autopsied Coronary Arteries
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea. wslee1227@dreamwiz.com
- KMID: 2028919
- DOI: http://doi.org/10.4070/kcj.2009.39.10.399
Abstract
- BACKGROUND AND OBJECTIVES
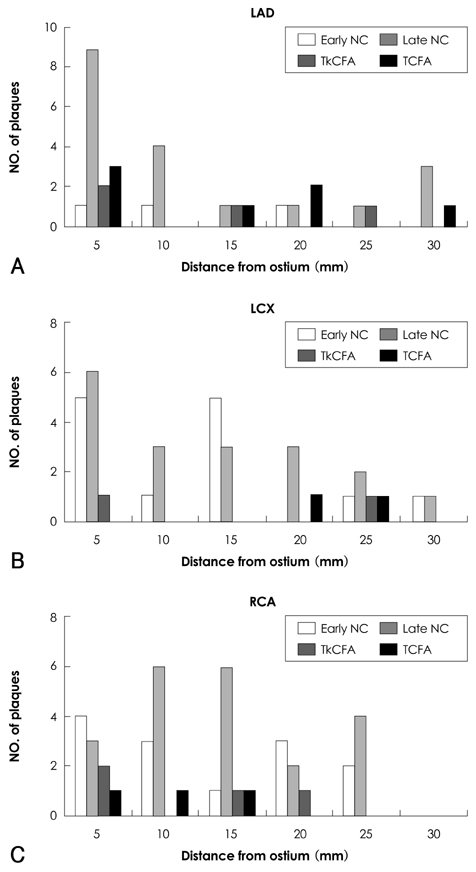
Virtual histology-intravascular ultrasound (VH-IVUS) studies on early-stage fibroatheroma, the probable precursor lesion of progression to thin-cap fibroatheroma (TCFA), have only rarely been done in man. We investigated the progression and observational frequency of fibroatheromas, and compared plaque components between early-stage and advance-staged fibroatheromas in the general population. SUBJECTS AND METHODS: We assessed coronary fibroatheromas using VH-IVUS and histopathologic analysis of 109 coronary lesions from 40 autopsied cases that were not due to sudden cardiac death (NSCD cases). Fibroatheromas were grouped into early fibroatheroma, late fibroatheroma, thick-cap fibroatheroma (TkCFA), and thin-cap fibroatheroma. RESULTS: Mean patient age was 45+/-11 years old and 71% were males. Of 109 lesions, 27% were early fibroatheromas, 53% late fibroatheromas, 9% TkCFA, and 11% TCFA. VH-IVUS showed that there was relatively less fibrotic and fibrofatty plaque and more dense calcium deposits as fibroatheromas progressed. Furthermore, the relative amounts of fibrotic and fibrofatty plaque decreased (r=0.773, p<0.001 and r=0.538, p<0.001, respectively) as the necrotic core increased, while the relative area of dense calcium increased (r=0.665, p<0.001) as the size of the necrotic core increased. CONCLUSION: Of NSCD cases in Korea, 27% were early fibroatheromas, 53% were late fibroatheromas, 9% were TkCFA, and 11% were TCFA. Advance-staged fibroatheromas show more necrotic core volume and more dense calcium than small, early-stage fibroatheromas.
MeSH Terms
Figure
Reference
-
1. Kolodgie FD, Narula J, Burke AP, et al. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000. 157:1259–1268.2. Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999. 82:269–272.3. Schmermund A, Schwartz RS, Adamzik M, et al. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with 'healthy' subjects dying out of hospital. Atherosclerosis. 2001. 155:499–508.4. Tardif JC, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006. 113:2936–2942.5. Park SH. Risk stratification of acute coronary syndrome. Korean Circ J. 2002. 32:735–755.6. Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007. 27:15–26.7. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007. 50:319–326.8. Kawasaki M, Bouma BE, Bressner J, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. 2006. 48:81–88.9. DeMaria AN, Narula J, Mahmud E, Tsimikas S. Imaging vulnerable plaque by ultrasound. J Am Coll Cardiol. 2006. 47:8 Suppl. C32–C39.10. Bae JH, Rihal CS, Lerman A. Tissue characterization of coronary plaques using intravascular ultrasound/virtual histology. Korean Circ J. 2006. 36:553–558.11. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002. 106:2200–2206.12. Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005. 25:2054–2061.13. Rodriguez-Granillo GA, Garcia-Garcia HM, Mc Fadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005. 46:2038–2042.14. Bae JH, Kwon TG, Kim KH, Hyun DW, Kim KY, Kim DS. Invivo coronary plaque composition in patients with acute coronary syndrome: a virtual histology intravascular ultrasound study. Korean Circ J. 2007. 37:437–442.15. Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007. 49:2073–2080.16. Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis: quid novi? Thromb Haemost. 2007. 97:873–879.17. Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004. 110:2843–2850.18. Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003. 349:2316–2325.19. Hong MK, Mintz GS, Lee CW, et al. Plaque ruptures in stable angina pectoris compared with acute coronary syndrome. Int J Cardiol. 2007. 114:78–82.20. Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004. 110:278–284.21. Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001. 16:285–292.22. Cheruvu PK, Finn AV, Gardner C, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007. 50:940–949.23. Kardys I, Vliegenthart R, Oudkerk M, Hofman A, Witteman JC. The female advantage in cardiovascular disease: do vascular beds contribute equally? Am J Epidemiol. 2007. 166:403–412.24. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007. 49:1230–1250.25. Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995. 92:1355–1374.26. Granada JF, Wallace-Bradley D, Win HK, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007. 27:387–393.27. Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.28. Nasir K, Shaw LJ, Liu ST, et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007. 50:953–960.29. Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation. 1996. 94:2662–2666.30. Kawasaki M, Takatsu H, Noda T, et al. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation. 2002. 105:2487–2492.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Non-Calcified Coronary Plaques Using 64-Slice Computed Tomography: Comparison With Intravascular Ultrasound
- Management of a Patient with Atheromatous Aortic Arch Diagnosed by as Intraoperative Transesophageal Echocardiography during Coronary Artery Bypass Grafting
- Noninvasive Detection of Coronary Atherosclerotic Plaques and Assessment of Stenosis Degree at Multidetector CT Coronary Angiography
- The Changes of Coronary Artery Stenosis by Sequential Coronary Angiographies
- Calcified Plaque of Coronary Artery: Factors Influencing Overestimation of Coronary Artery Stenosis on Coronary CT Angiography