Korean J Obstet Gynecol.
2011 Apr;54(4):192-198. 10.5468/KJOG.2011.54.4.192.
Is the expression of p16INK4A and galectin-3 correlated with disease progression of cervical neoplasia?
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Hallym University College of Medicine, Seoul, Korea. vth2000@naver.com
- KMID: 2013235
- DOI: http://doi.org/10.5468/KJOG.2011.54.4.192
Abstract
OBJECTIVE
The aim of this study is to investigate whether the expressions of p16INK4A and galectin-3 are associated with the progression of cervical neoplasia and to evaluate its usefulness as a diagnostic tool.
METHODS
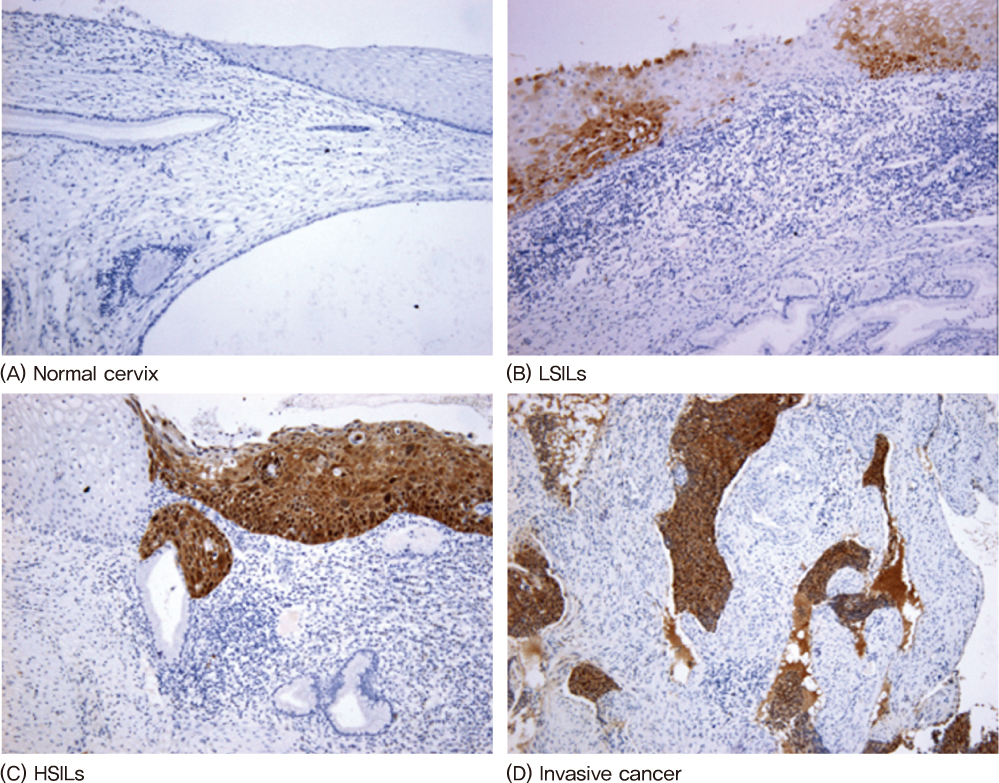
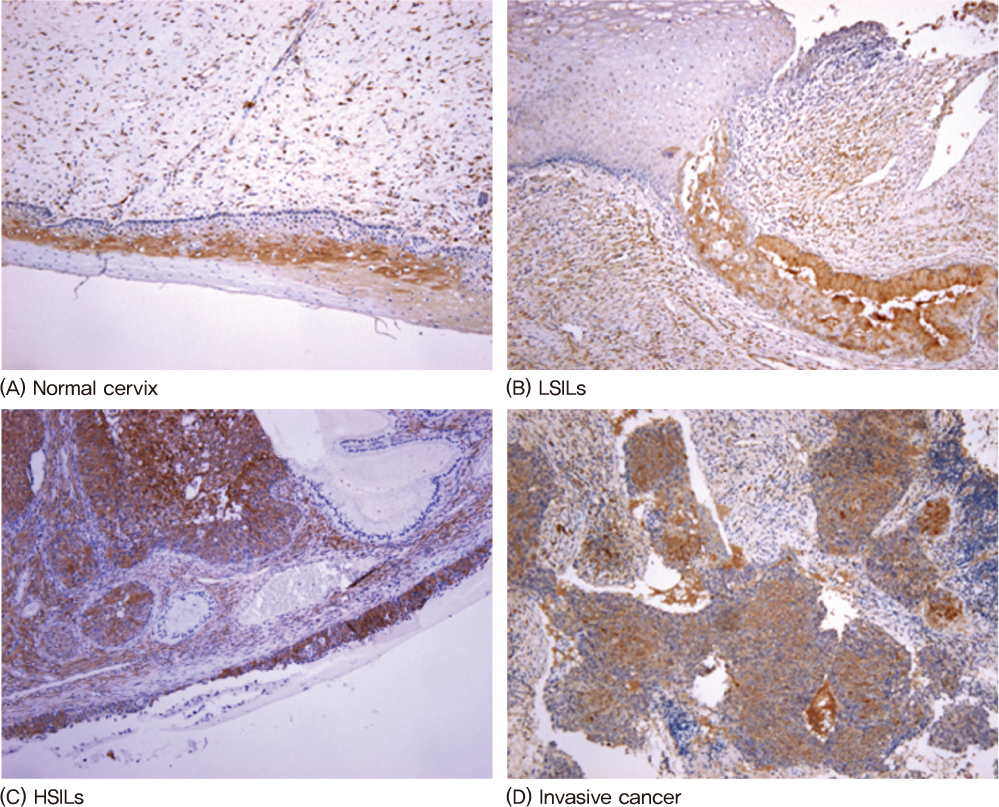
Eighty-seven formalin-fixed paraffin-embedded cervical specimens (20 normal, 17 LSILs, 26 HSILs, 24 invasive cervical cancers) collected between 2005 and 2009 were selected. We examined the expression of p16INK4A and galectin-3 using immunohistochemical stains with the scoring system.
RESULTS
The mean proportion of p16INK4A and galectin-3 was 0.1 and 1.70 in normal lesions, 1.35 and 2.17 in LSILs, 3.42 and 3.11 in HSILs, 3.79 and 3.08 in invasive cancers. The mean proportion of p16INK4A and galectin-3 was correlated significantly with the degree of neoplasia (P<0.05).
CONCLUSION
The increased immunohistochemical staining expressions of the p16INK4A and galectin-3 are associated with the progression of cervical neoplasia. Therefore immunohistochemical staining of p16INK4A and galectin-3 can be a useful biomarker for the diagnosis of a progression of cervical neoplasia.
MeSH Terms
Figure
Reference
-
1. National Cancer Information Center [Internet]. c2008. cited 2010 May 31. Goyang (KR): National Cancer Information Center;Available from: http://www.cancer.go.kr/cms/index.html.2. Anderson GH, Boyes DA, Benedet JL, Le Riche JC, Matisic JP, Suen KC, et al. Organisation and results of the cervical cytology screening programme in British Columbia, 1955-85. Br Med J (Clin Res Ed). 1988. 296:975–978.3. Sasieni PD, Cuzick J, Lynch-Farmery E. The National Co-ordinating Network for Cervical Screening Working Group. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br J Cancer. 1996. 73:1001–1005.4. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995. 141:680–689.5. Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993. 75:495–505.6. Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996. 93:4350–4354.7. Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994. 269:20807–20810.8. Konstantinov KN, Robbins BA, Liu FT. Galectin-3, a beta-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. Am J Pathol. 1996. 148:25–30.9. Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1987. 84:6452–6456.10. Woo HJ, Shaw LM, Messier JM, Mercurio AM. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem. 1990. 265:7097–7099.11. Inohara H, Akahani S, Koths K, Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996. 56:4530–4534.12. Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992. 148:861–867.13. Liu FT. S-type mammalian lectins in allergic inflammation. Immunol Today. 1993. 14:486–490.14. Inohara H, Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 1995. 55:3267–3271.15. Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998. 153:1741–1748.16. Nakao Y, Yang X, Yokoyama M, Ferenczy A, Tang SC, Pater MM, et al. Induction of p16 during immortalization by HPV 16 and 18 and not during malignant transformation. Br J Cancer. 1997. 75:1410–1416.17. Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992. 258:424–429.18. Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990. 17:489–503.19. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001. 92:276–284.20. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001. 25:884–891.21. Negri G, Egarter-Vigl E, Kasal A, Romano F, Haitel A, Mian C. p16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors: an immunohistochemical study with immunocytochemical correlations. Am J Surg Pathol. 2003. 27:187–193.22. Kim JR, Kim SY, Kim MJ, Kim JH. Alterations of CDKN2 (MTS1/p16INK4A) gene in paraffin-embedded tumor tissues of human stomach, lung, cervix and liver cancers. Exp Mol Med. 1998. 30:109–114.23. Wong YF, Wang W, Wong FWS, Chanc AMZ. Genetic studies in gynecologic malignancies. J Pract Gynecol Oncol. 1993. 9:101–102.24. Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995. 75:2818–2826.25. Lee JW, Song SY, Choi JJ, Choi CH, Kim TJ, Kim J, et al. Decreased galectin-3 expression during the progression of cervical neoplasia. J Cancer Res Clin Oncol. 2006. 132:241–247.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decreased expression of galectin-3 is associated with the progression of cervical neoplasia
- p16INK4a immunohistochemistry is a promising biomarker to predict the outcome of low grade cervical intraepithelial neoplasia: comparison study with HPV genotyping
- VEGF Expression and Angiogenesis in Uterine Cervical Carcinomas
- The Usefulness of p16INK4a Immunocytochemical Staining in ASC-H Patients
- Human Papillomavirus Genotyping and p16INK4a Expression in Cervical Lesions: A Combined Test to Avoid Cervical Cancer Progression