J Korean Soc Magn Reson Med.
2014 Sep;18(3):200-207. 10.13104/jksmrm.2014.18.3.200.
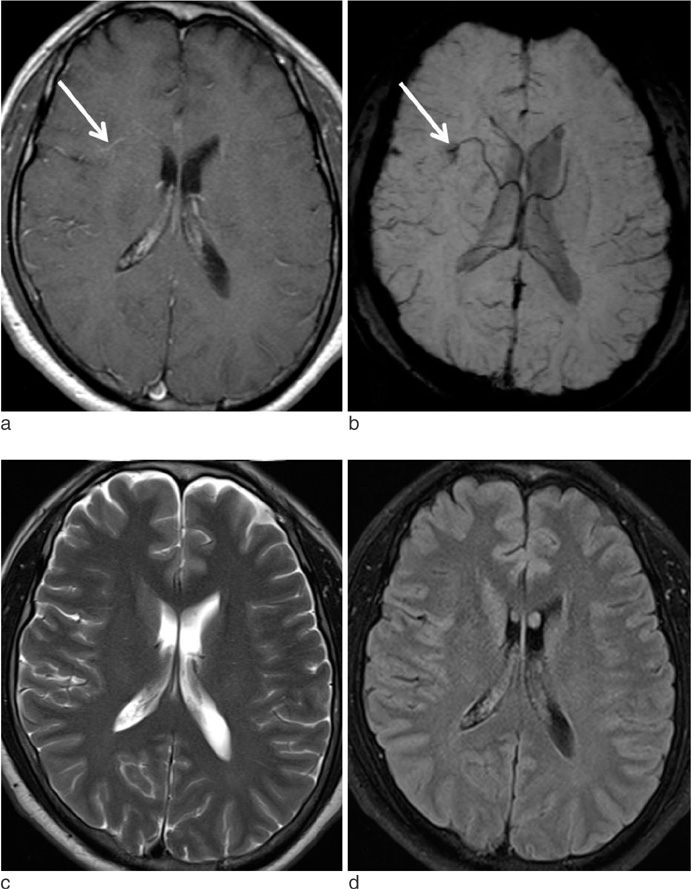
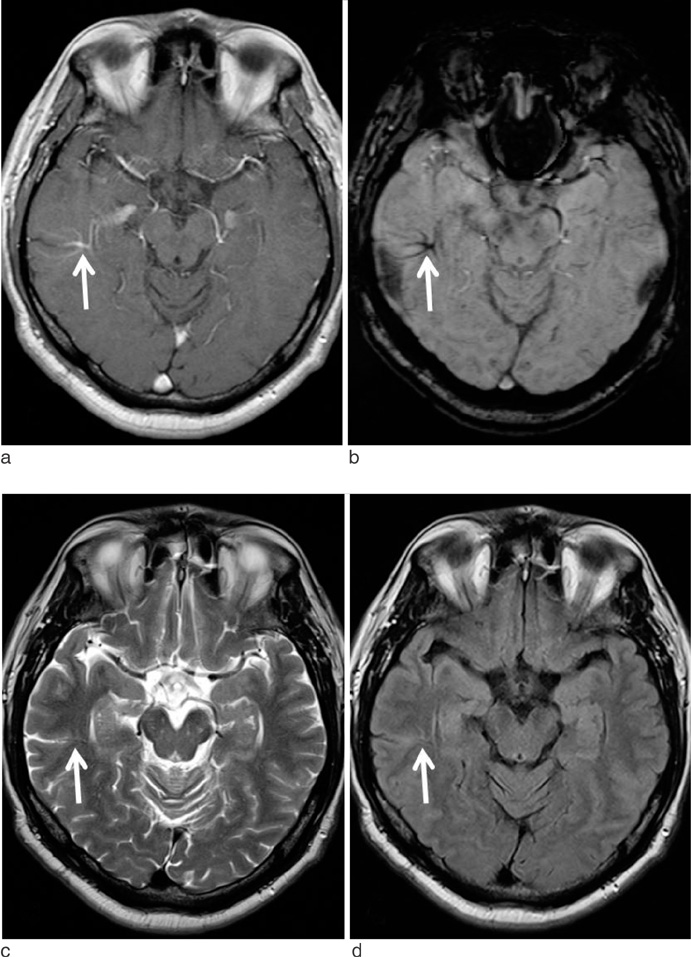
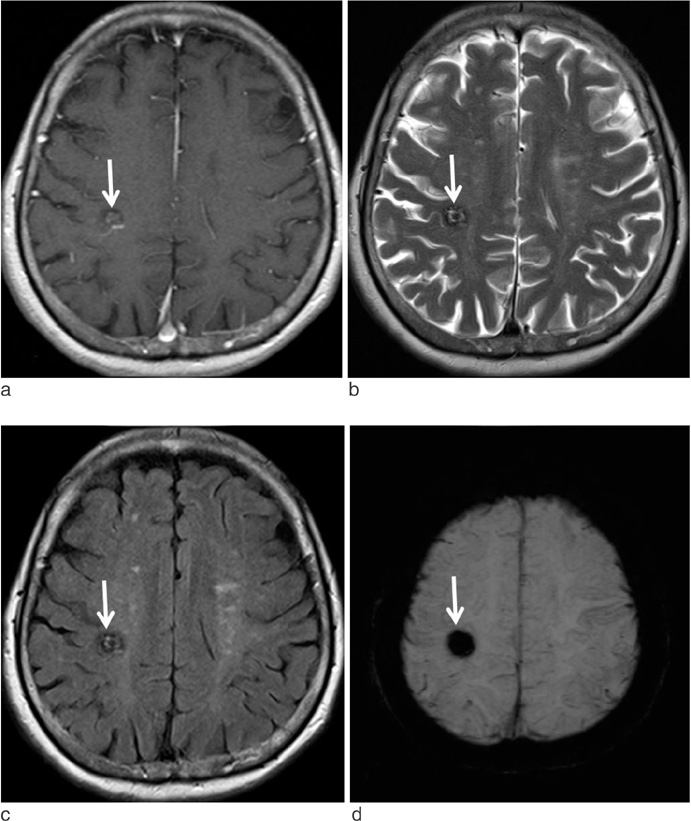
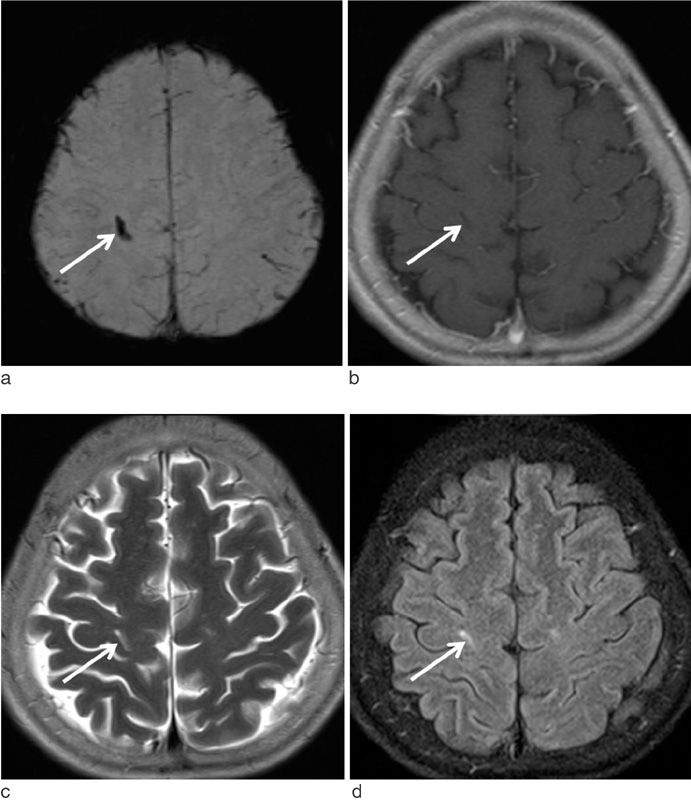
Susceptibility-Weighted MR Imaging for the Detection of Developmental Venous Anomaly: Comparison with T2 and FLAIR Imaging
- Affiliations
-
- 1Department of Radiology, Gyeongsang National University School of Medicine, Jinju, Korea. choids@gnu.ac.kr
- 2Gyeongsang Institute of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Neurology, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 1999919
- DOI: http://doi.org/10.13104/jksmrm.2014.18.3.200
Abstract
- PURPOSE
We evaluated the diagnostic value of susceptibility-weighted imaging (SWI) for the detection of developmental venous anomaly (DVA).
MATERIALS AND METHODS
Retrospective review of 1068 brain MR examinations found 28 DVAs in 28 patients (2.6%) on contrast-enhanced T1-weighted images. SWI, T2, and FLAIR images of 28 patients with DVA and 28 sex- and age-matched control patients without DVA were analyzed by blinded readers on each type of sequences. All images were independently reviewed by two radiologists who were blinded to other MR imaging finding. In cases of discrepancy, two reviewers reached a consensus later. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each MR sequence for the detection of DVA were determined. Statistical analysis was performed by using the Mcnemar test. The significance level was p < 0.05.
RESULTS
The sensitivity, specificity, PPV, and NPV of SWI for the detection of DVA were 85.7%, 92.9%, 92.3%, and 86.7%, respectively. T2 and FLAIR images showed sensitivity of 35.7% and 35.7%, specificity of 92.9% and 96.4%, PPV of 83.3% and 90.9%, and NPV of 59.1% and 60.0%, respectively. On SWI, the sensitivity and NPV for the detection of DVAs were significantly higher than those of T2 and FLAIR images (p < 0.05).
CONCLUSION
SWI was sensitive and specific for the detection of DVA.
Keyword
MeSH Terms
Figure
Reference
-
1. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986; 9:233–242.2. Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Arch Neurol. 1978; 35:323–325.3. Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol. 1992; 159:1299–1307.4. Takasugi M, Fujii S, Shinohara Y, Kaminou T, Watanabe T, Ogawa T. Parenchymal hypointense foci associated with developmental venous anomalies: evaluation by phase-sensitive MR imaging at 3T. AJNR Am J Neuroradiol. 2013; 34:1940–1944.5. Santucci GM, Leach JL, Ying J, Leach SD, Tomsick TA. Brain parenchymal signal abnormalities associated with developmental venous anomalies: detailed MR imaging assessment. AJNR Am J Neuroradiol. 2008; 29:1317–1323.6. San Millán Ruíz D, Delavelle J, Yilmaz H, et al. Parenchymal abnormalities associated with developmental venous anomalies. Neuroradiology. 2007; 49:987–995.7. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014; 69:661–668.8. Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. 2012; 259:1426–1432.9. Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009; 64:74–83.10. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004; 52:612–618.11. Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009; 30:232–252.12. Tsui YK, Tsai FY, Hasso AN, Greensite F, Nguyen BV. Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci. 2009; 287:7–16.13. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009; 30:19–30.14. McCormick WF, Boulter TR. Vascular malformations ("angiomas") of the dura mater. J Neurosurg. 1966; 25:309–311.15. Ruiz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: current concepts. Ann Neurol. 2009; 66:271–283.16. San Millan Ruiz D, Gailloud P. Cerebral developmental venous anomalies. Childs Nerv Syst. 2010; 26:1395–1406.17. Garner TB, Curling OD Jr, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991; 75:715–722.18. Lee BC, Vo KD, Kido DK, et al. MR high-resolution blood oxygenation level dependent venography of occult (low-flow) vascular lesions. AJNR Am J Neuroradiol. 1999; 20:1239–1242.19. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005; 22:439–450.20. Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008; 29:9–17.21. Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted imaging of brain-A pictorial review. Neuroradiology. 2008; 50:105–116.22. Huber G, Henkes H, Hermes M, et al. Regional association of developmental venous anomalies with angiographically occult vascular malformations. Eur Radiol. 1996; 6:30–37.23. de Souza JM, Domingues RC, Cruz LC Jr, et al. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with T2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008; 29:154–158.24. de Champfleur NM, Langlois C, Ankenbrandt WJ, et al. Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery. 2011; 68:641–647.25. McLaughlin MR, Kondziolka D, Flickinger JC, Lunsford S, Lunsford LD. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998; 43:195–200.26. Jung HN, Kim ST, Cha J, et al. Diffusion and perfusion MRI findings of the signal-intensity abnormalities of brain associated with developmental venous anomaly. AJNR Am J Neuroradiol. 2014; 03. 20. [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR Imaging with FLAIR Pulse Sequence in Various Cerebral Lesions: Comparison with T2-Weighted Imaging
- Associated Brain Parenchymal Abnormalities in Developmental Venous Anomalies: Evaluation with Susceptibility-weighted MR Imaging
- A Comparison of Lesion Detection and Conspicuity on T2-weighted Images (T2 FFE), FLAIR and Diffusion-weighted Images in Patients with Traumatic Brain Injury
- Usefulness of Fluid Attenuated Inve rsion Re c overy(FLAIR) Image
- Detection of Acute Intraventricular Hemorrhage: Comparison of FLAIR MR Imaging with Unenhanced CT