Korean J Urol.
2014 Feb;55(2):81-90. 10.4111/kju.2014.55.2.81.
Neural Mechanisms Underlying Lower Urinary Tract Dysfunction
- Affiliations
-
- 1Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. nyos@pitt.edu
- 2Department of Urology, Oakland University William Beaumont School of Medicine, Royal Oak, MI, USA.
- KMID: 1988431
- DOI: http://doi.org/10.4111/kju.2014.55.2.81
Abstract
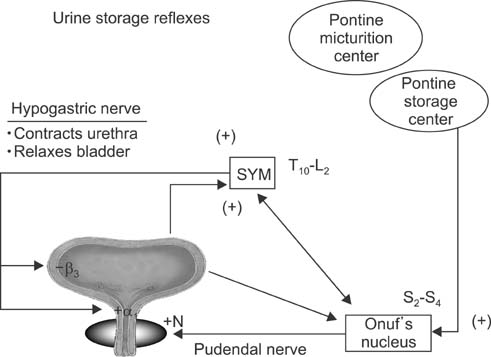
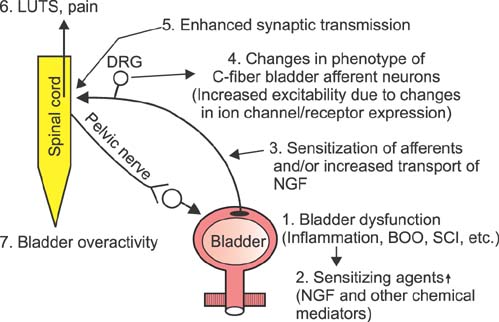
- This article summarizes anatomical, neurophysiological, and pharmacological studies in humans and animals to provide insights into the neural circuitry and neurotransmitter mechanisms controlling the lower urinary tract and alterations in these mechanisms in lower urinary tract dysfunction. The functions of the lower urinary tract, to store and periodically release urine, are dependent on the activity of smooth and striated muscles in the bladder, urethra, and external urethral sphincter. During urine storage, the outlet is closed and the bladder smooth muscle is quiescent. When bladder volume reaches the micturition threshold, activation of a micturition center in the dorsolateral pons (the pontine micturition center) induces a bladder contraction and a reciprocal relaxation of the urethra, leading to bladder emptying. During voiding, sacral parasympathetic (pelvic) nerves provide an excitatory input (cholinergic and purinergic) to the bladder and inhibitory input (nitrergic) to the urethra. These peripheral systems are integrated by excitatory and inhibitory regulation at the levels of the spinal cord and the brain. Therefore, injury or diseases of the nervous system, as well as disorders of the peripheral organs, can produce lower urinary tract dysfunction, leading to lower urinary tract symptoms, including both storage and voiding symptoms, and pelvic pain. Neuroplasticity underlying pathological changes in lower urinary tract function is discussed.
MeSH Terms
-
Animals
Brain
Humans
Lower Urinary Tract Symptoms
Muscle, Smooth
Muscle, Striated
Nerve Growth Factor
Nervous System
Neuronal Plasticity
Neurotransmitter Agents
Pelvic Pain
Pons
Relaxation
Spinal Cord
Urethra
Urinary Bladder
Urinary Bladder, Overactive
Urinary Tract*
Urination
Nerve Growth Factor
Neurotransmitter Agents
Figure
Cited by 1 articles
-
Changes in autonomic nervous system activity after treatment with alpha-blocker in men with lower urinary tract symptoms
Kang Hee Shim, Tae Woo Kim, Byung Ha Chung, Sung Won Lee, Jong Kwan Park, Kwangsung Park, Jun Cheon, Kyung Seop Lee, Hyung-Jee Kim, Do-Hwan Seong, Seung-June Oh, Sae Woong Kim, Ji Youl Lee, Seol Ho Choo, Jong Bo Choi
Investig Clin Urol. 2018;59(1):49-54. doi: 10.4111/icu.2018.59.1.49.
Reference
-
1. Fry C, Brading AF, Hussain M. Cell biology. In : Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd.;2005. p. 313–362.2. Morrison JF, Birder L, Craggs M. Neural control. In : Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd.;2005. p. 363–422.3. de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In : Maggi CA, editor. Nervous control of the urogenital system: autonomic nervous system. London: Harwood Academic Publishers;1993. p. 227–290.4. Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2008; 377:437–448.5. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004; 56:581–631.6. Yoshimura N, Chancellor MB. Physiology and pharmacology of the bladder and urethra. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders;2011. p. 1786–1833.7. Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981; 201:415–440.8. Morgan C, deGroat WC, Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986; 243:23–40.9. Thor KB, Morgan C, Nadelhaft I, Houston M, De Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989; 288:263–279.10. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004; 84:935–986.11. Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002; 59:5 Suppl 1. 43–50.12. Ouslander JG. Management of overactive bladder. N Engl J Med. 2004; 350:786–799.13. De Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol. 1972; 226:289–309.14. de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol. 1976; 259:223–237.15. Nishizawa O, Fukuda T, Matsuzaki A, Moriya I, Harada T, Tsuchida S. Role of the sympathetic nerve in bladder and urethral sphincter function during the micturition cycle in the dog evaluated by pressure flow EMG study. J Urol. 1985; 134:1259–1261.16. Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982; 127:958–963.17. De Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975; 87:201–211.18. de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981; 3:135–160.19. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990; 30:Suppl. S71–S77.20. Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997; 17:8402–8407.21. Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999; 57:583–606.22. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006; 152:59–84.23. de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol Urodyn. 2010; 29:63–76.24. de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012; 235:123–132.25. de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969; 200:87–108.26. Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol. 1999; 277(3 Pt 2):R786–R794.27. Geirsson G, Lindstrom S, Fall M. The bladder cooling reflex in man: characteristics and sensitivity to temperature. Br J Urol. 1993; 71:675–680.28. Fall M, Lindstrom S, Mazieres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990; 427:281–300.29. Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol. 2004; 172:1175–1178.30. Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005; 65:400–405.31. Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005; 174:977–982.32. Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, et al. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004; 46:247–253.33. Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004; 93:770–776.34. de Groat WC. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Paraplegia. 1995; 33:493–505.35. Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995; 54:215–224.36. Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997; 503(Pt 2):269–276.37. Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, et al. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 α-subunit in rats with spinal cord injury. J Urol. 2013; 190:2296–2304.38. Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, et al. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol. 2005; 288:F466–F473.39. Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004; 171:478–482.40. Zhang X, Douglas KL, Jin H, Eldaif BM, Nassar R, Fraser MO, et al. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R2084–R2096.41. Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, et al. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008; 179:1178–1183.42. Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009; 16:660–668.43. Miyazato M, Sugaya K, Saito S, Chancellor MB, Goins WF, Goss JR, et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010; 184:1204–1210.44. Santos-Silva A, Charrua A, Cruz CD, Gharat L, Avelino A, Cruz F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton Neurosci. 2012; 166:35–38.45. Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, et al. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011; 300:F1223–F1234.46. Giannantoni A, Di Stasi SM, Nardicchi V, Zucchi A, Macchioni L, Bini V, et al. Botulinum-A toxin injections into the detrusor muscle decrease nerve growth factor bladder tissue levels in patients with neurogenic detrusor overactivity. J Urol. 2006; 175:2341–2344.47. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol. 2009; 56:700–706.48. Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res. 2006; 152:97–115.49. Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006; 26:10847–10855.50. Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004; 5:150–156.51. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007; 7:9.52. Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002; 168:2269–2274.53. Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000; 163:1349–1356.54. Steers WD, De Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol. 1988; 140:864–871.55. Chai TC, Gray ML, Steers WD. The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. J Urol. 1998; 160:34–38.56. Hirayama A, Fujimoto K, Matsumoto Y, Ozono S, Hirao Y. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003; 62:909–913.57. Hirayama A, Fujimoto K, Matsumoto Y, Hirao Y. Nocturia in men with lower urinary tract symptoms is associated with both nocturnal polyuria and detrusor overactivity with positive response to ice water test. Urology. 2005; 65:1064–1069.58. Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996; 155:379–385.59. Speakman MJ, Brading AF, Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA. Bladder outflow obstruction--a cause of denervation supersensitivity. J Urol. 1987; 138:1461–1466.60. Boselli C, Govoni S, Condino AM, D'Agostino G. Bladder instability: a re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Auton Pharmacol. 2001; 21:219–229.61. O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001; 87:617–622.62. Haferkamp A, Mundhenk J, Bastian PJ, Reitz A, Dorsam J, Pannek J, et al. Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol. 2004; 46:799–805.63. Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, et al. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003; 284:R1241–R1248.64. Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, et al. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008; 27:330–340.65. Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, et al. Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn. 2011; 30:1639–1645.66. Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006; 97:612–616.67. Kubota Y, Biers SM, Kohri K, Brading AF. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006; 25:205–210.68. Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest. 1991; 88:1709–1715.69. Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008; 72:104–108.70. Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology. 2011; 78:721.e1–721.e6.71. Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002; 59:5 Suppl 1. 61–67.72. Yoshimura N, Birder L. Interstitial cystitis and related painful bladder syndromes: pathophysiology. In : Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic abdominal and visceral pain: theory and practice. New York: Informa Healthcare USA;2007. p. 495–520.73. Birder LA. Urothelial signaling. Handb Exp Pharmacol. 2011; (202):207–231.74. Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999; 19:4644–4653.75. Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, et al. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R1661–R1670.76. Lazzeri M, Beneforti P, Benaim G, Maggi CA, Lecci A, Turini D. Intravesical capsaicin for treatment of severe bladder pain: a randomized placebo controlled study. J Urol. 1996; 156:947–952.77. Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000; 164(3 Pt 1):676–679.78. Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol. 2005; 173:1590–1594.79. Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol. 2006; 176:797–801.80. Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004; 24:11253–11263.81. Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, Cruz F, et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009; 181:379–386.82. Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007; 177:1537–1541.83. Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999; 161:438–441.84. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997; 79:572–577.85. Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997; 78:449–459.86. Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001; 165:975–979.87. Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011; 185:1716–1721.88. Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013; 190:757–764.89. Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007; 26:814–819.90. Lee WC, Wu HP, Tai TY, Yu HJ, Chiang PH. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J Urol. 2009; 181:198–203.91. Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997; 157:580–584.92. Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005; 95:733–738.93. Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995; 153:342–344.94. Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009; 182:6 Suppl. S18–S26.95. Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005; 173:1027–1032.96. Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol. 2004; 171:1959–1964.97. Steinbacher BC Jr, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998; 782(1-2):255–260.98. Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, et al. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002; 168:1259–1264.99. Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996; 2:703–707.100. Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997; 46:Suppl 2. S43–S49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Benign Prostate Hyperplasia and Erectile Dysfunction: What is Reality?
- Pathophysiology of lower urinary tract dysfunction in the older patient
- Management of Lower Urinary Tract Dysfunction in Patients with Neurological Disorders
- Geriatric considerations in the diagnosis and management of lower urinary tract dysfunction
- Overview of the Epidemiology of Lower Urinary Tract Dysfunction in South Korea