J Clin Neurol.
2013 Oct;9(4):252-258. 10.3988/jcn.2013.9.4.252.
Contribution of Galvanic Vestibular Stimulation for the Diagnosis of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
- Affiliations
-
- 1Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. deniseg@medicina.ufmg.br
- 2Faculty of Engineering, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
- 3Fundacao Hemominas, State Centre of Haematology and Haemotherapy, Belo Horizonte, Brazil.
- KMID: 1980545
- DOI: http://doi.org/10.3988/jcn.2013.9.4.252
Abstract
- BACKGROUND AND PURPOSE
Galvanic vestibular stimulation (GVS) is a low-cost and safe examination for testing the vestibulospinal pathway. Human T-lymphotropic virus 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a slowly progressive disease that affects the vestibulospinal tract early in its course. This study compared the electromyographic (EMG) responses triggered by GVS of asymptomatic HTLV-1-infected subjects and subjects with HAM/TSP.
METHODS
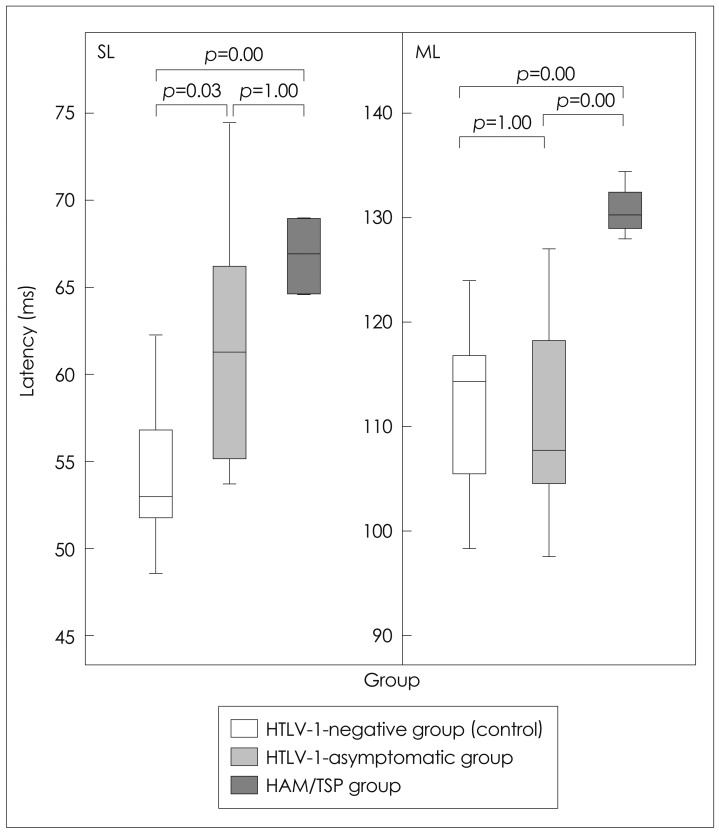
Bipolar galvanic stimuli (400 ms and 2 mA) were applied to the mastoid processes of 39 subjects (n=120 stimulations per subject, with 60 from each lower limb). Both the short latency (SL) and medium latency (ML) components of the EMG response were recorded from the soleus muscles of 13 healthy, HTLV-1-negative adults (56+/-5 years, mean+/-SD), and 26 individuals infected with HTLV-1, of whom 13 were asymptomatic (56+/-8 years) and 13 had HAM/TSP (60+/-6 years).
RESULTS
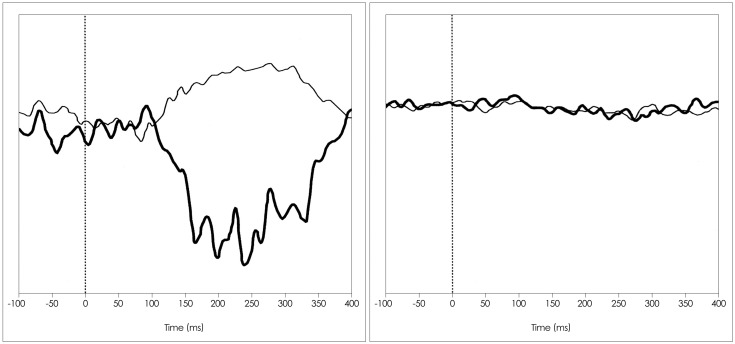
The SL and ML EMG components were 55+/-4 and 112+/-10 ms, respectively, in the group of healthy subjects, 61+/-6 and 112+/-10 ms and in the HTLV-1-asymptomatic group, and 67+/-8 and 130+/-3 ms in the HAM/TSP group (p=0.001). The SL component was delayed in 4/13 (31%) of the examinations in the HTLV-1-asymptomatic group, while the ML component was normal in all of them. In the HAM/TSP group, the most common alteration was the absence of waves.
CONCLUSIONS
A pattern of abnormal vestibular-evoked EMG responses was found in HTLV-1-neurological disease, ranging from delayed latency among asymptomatic carriers to the absence of a response in HAM/TSP. GVS may contribute to the early diagnosis and monitoring of nontraumatic myelopathies.
Keyword
MeSH Terms
Figure
Reference
-
1. Watson SR, Colebatch JG. Vestibular-evoked electromyographic responses in soleus: a comparison between click and galvanic stimulation. Exp Brain Res. 1998; 119:504–510. PMID: 9588785.
Article2. Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993; 94:143–151. PMID: 8335069.
Article3. Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994; 478(Pt 2):363–372. PMID: 7965852.
Article4. Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984; 51:1236–1256. PMID: 6737029.
Article5. Muto N, Shinomiya K, Komori H, Mochida K, Furuya K. Spinal cord monitoring of the ventral funiculus function Analysis of spinal field potentials after galvanic vestibular stimulation. Spine (Phila Pa 1976). 1995; 20:2429–2434. discussion 2435. PMID: 8578394.6. Séverac Cauquil A, Martinez P, Ouaknine M, Tardy-Gervet MF. Orientation of the body response to galvanic stimulation as a function of the inter-vestibular imbalance. Exp Brain Res. 2000; 133:501–505. PMID: 10985684.
Article7. Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004; 96:2301–2316. PMID: 15133017.
Article8. Day BL, Séverac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997; 500(Pt 3):661–672. PMID: 9161984.
Article9. Baldissera F, Cavallari P, Tassone G. Effects of transmastoid electrical stimulation on the triceps brachii EMG in man. Neuroreport. 1990; 1:191–193. PMID: 2129879.
Article10. Iles JF, Ali AS, Savic G. Vestibular-evoked muscle responses in patients with spinal cord injury. Brain. 2004; 127(Pt 7):1584–1592. PMID: 15128616.
Article11. Watson SR, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalogr Clin Neurophysiol. 1997; 105:476–483. PMID: 9448650.
Article12. Muise SB, Lam CK, Bent LR. Reduced input from foot sole skin through cooling differentially modulates the short latency and medium latency vestibular reflex responses to galvanic vestibular stimulation. Exp Brain Res. 2012; 218:63–71. PMID: 22278107.
Article13. Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005; 563(Pt 1):229–234. PMID: 15618274.
Article14. Welgampola MS, Colebatch JG. Selective effects of ageing on vestibular-dependent lower limb responses following galvanic stimulation. Clin Neurophysiol. 2002; 113:528–534. PMID: 11955997.
Article15. Liechti M, Müller R, Lam T, Curt A. Vestibulospinal responses in motor incomplete spinal cord injury. Clin Neurophysiol. 2008; 119:2804–2812. PMID: 18842452.
Article16. Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005; 24:6058–6068. PMID: 16155612.
Article17. Izumo S. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP). Neuropathology. 2010; [Epub ahead of print].
Article18. De Castro-Costa CM, Araújo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses. 2006; 22:931–935. PMID: 17067261.19. Felipe L, Gonçalves DU, Santos MA, Proietti FA, Ribas JG, Carneiro-Proietti AB, et al. Vestibular-evoked myogenic potential (VEMP) to evaluate cervical myelopathy in human T-cell lymphotropic virus type I infection. Spine (Phila Pa 1976). 2008; 33:1180–1184. PMID: 18469690.
Article20. Allain JP, Stramer SL, Carneiro-Proietti AB, Martins ML, Lopes da Silva SN, Ribeiro M, et al. Transfusion-transmitted infectious diseases. Biologicals. 2009; 37:71–77. PMID: 19231236.
Article21. Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010; 23:577–589. PMID: 20610824.
Article22. Proietti FA, Lima-Martins MV, Passos VM, Brener S, Carneiro-Proietti AB. HTLV-I/II seropositivity among eligible blood donors from Minas Gerais State, Brasil. Vox Sang. 1994; 67:77. PMID: 7975458.
Article23. Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983; 117:307–309. PMID: 6603098.
Article24. Hashimoto T, Uozumi T, Tsuji S. Paraspinal motor evoked potentials by magnetic stimulation of the motor cortex. Neurology. 2000; 55:885–888. PMID: 10994018.
Article25. Goncalves DU, Proietti FA, Barbosa-Stancioli EF, Martins ML, Ribas JG, Martins-Filho OA, et al. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network. Inflamm Allergy Drug Targets. 2008; 7:98–107. PMID: 18691139.
Article26. Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990; 3:1096–1101. PMID: 2213510.27. Peterson BW, Abzug C. Properties of projections from vestibular nuclei to medial reticular formation in the cat. J Neurophysiol. 1975; 38:1421–1435. PMID: 1221080.
Article28. Murofushi T, Shimizu K, Takegoshi H, Cheng PW. Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch Otolaryngol Head Neck Surg. 2001; 127:1069–1072. PMID: 11556854.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of Human T Lymphotropic Virus Type I Associated Myelopathy (HAM)
- The study of galvanic vestibular stimulation in patients of total unilateral vestibular loss
- A case of HTLV-I associated myelopathy(HAM) in Korea
- The Optimization of Galvanic Vestibular Stimulation according to Evoked Nystagmus
- Nucleotide sequence analysis of HTLV-I isolate from a Korean patient with HAM/TSP