J Adv Prosthodont.
2010 Dec;2(4):142-147. 10.4047/jap.2010.2.4.142.
A histomorphometric study of dental implants with different surface characteristics
- Affiliations
-
- 1Department of Prosthodontics, Graduate School, Seoul National University, Seoul, Korea. jhoyang@snu.ac.kr
- KMID: 1975189
- DOI: http://doi.org/10.4047/jap.2010.2.4.142
Abstract
- PURPOSE
One of the major keys to achieve successful osseointegration of the implant is its surface properties. The aim of this study was to investigate the bone response to dental implants with different surface characteristics using the rabbit tibia model. Tricalcium phosphate (TCP) coated, anodic oxidized and turned (control) surfaces were compared.
MATERIALS AND METHODS
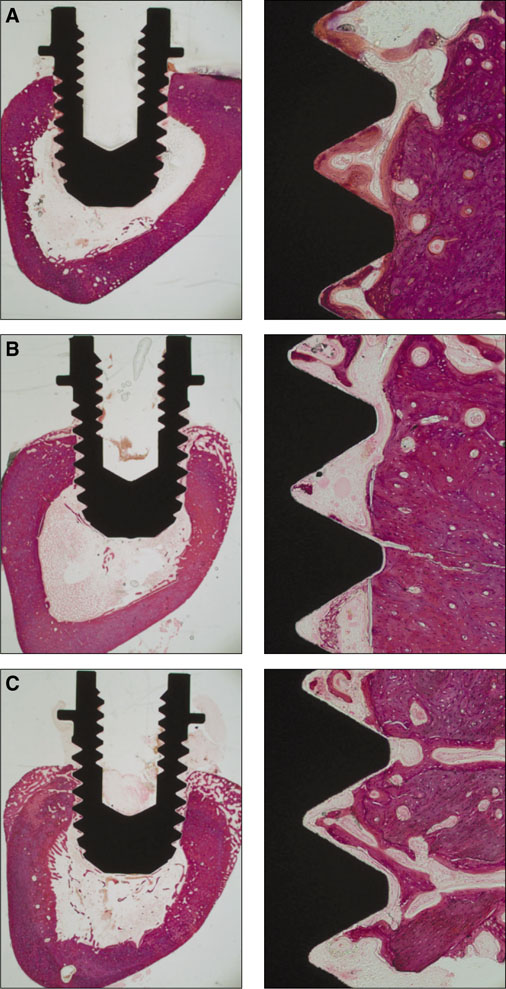
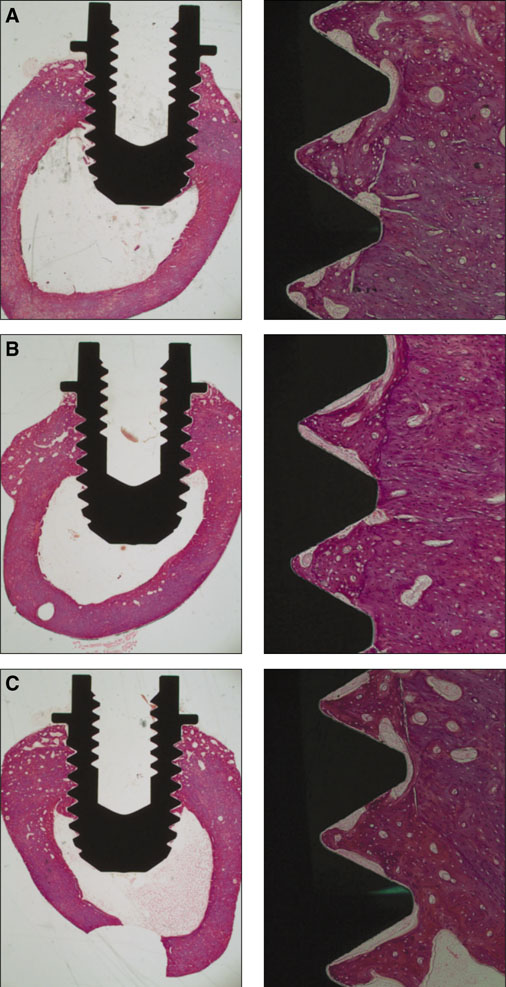
Seventy two implants were placed in the tibia of eighteen rabbits. Nine rabbits were sacrificed at 3 weeks of healing and the remaining nine were sacrificed at 6 weeks of healing. The bone-to-implant contact (BIC) and the bone volume density (BVD) were assessed by light microscope after 3 and 6 weeks of healing.
RESULTS
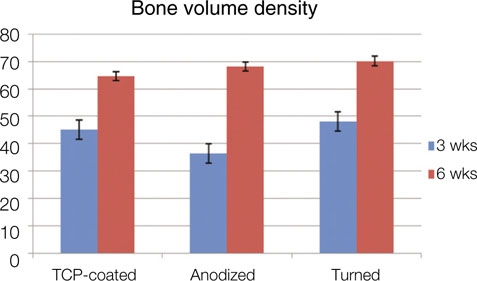
Statistical analysis showed that no significant differences in the BIC and BVD were observed between the different implant surfaces and the control group at 3 weeks and 6 weeks of healing. Data also suggested that the BVD of all the surfaces showed significant difference at 3 and 6 weeks.
CONCLUSION
The present study has showed that osseointegration occurred in all investigated types of surface-treated implants. In the current study all of the threads of the implants were observed to calculate BIC and BVD values (instead of choosing some of the threads from the bone cortex for example), which didn't make BIC or BVD percentage values better than in the control group, therefore the clinical relevance of these results remains to be shown.
Keyword
MeSH Terms
Figure
Reference
-
1. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981. 52:155–170.2. Kasemo B, Lausmaa J. Surface science aspects on inorganic biomaterials. CRC Crit Rev Clin Neurobiol. 1986. 4:335–380.3. Kim YH, Koak JY, Chang IT, Wennerberg A, Heo SJ. A histomorphometric analysis of the effects of various surface treatment methods on osseointegration. Int J Oral Maxillofac Implants. 2003. 18:349–356.4. Pilliar RM. Overview of surface variability of metallic endosseous dental implants: textured and porous surface-structured designs. Implant Dent. 1998. 7:305–314.5. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000. 84:522–534.6. Masuda T, Yliheikkilä PK, Felton DA, Cooper LF. Generalizations regarding the process and phenomenon of osseointegration. Part I. In vivo studies. Int J Oral Maxillofac Implants. 1998. 13:17–29.7. Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, Boyan BD. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996. 32:55–63.8. Larsson C, Thomsen P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994. 15:1062–1074.9. Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008. 87:303–311.10. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992. 7:302–310.11. Schwartz Z, Martin JY, Dean DD, Simpson J, Cochran DL, Boyan BD. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996. 30:145–155.12. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995. 29:389–401.13. Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001. 58:570–592.14. Park EK, Lee YE, Choi JY, Oh SH, Shin HI, Kim KH, Kim SY, Kim S. Cellular biocompatibility and stimulatory effects of calcium metaphosphate on osteoblastic differentiation of human bone marrow-derived stromal cells. Biomaterials. 2004. 25:3403–3411.15. Fini M, Cigada A, Rondelli G, Chiesa R, Giardino R, Giavaresi G, Nicoli Aldini N, Torricelli P, Vicentini B. In vitro and in vivo behaviour of Ca- and P-enriched anodized titanium. Biomaterials. 1999. 20:1587–1594.16. Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000. 15:675–690.17. Ellingsen JE. Surface configurations of dental implants. Periodontol 2000. 1998. 17:36–46.18. Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, Ericson LE. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996. 17:605–616.19. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–326.20. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998. 11:491–501.21. Larsson C, Emanuelsson L, Thomsen P, Ericson LE, Aronsson BO, Kasemo B, Lausmaa J. Bone response to surface modified titanium implants - studies on the tissue response after 1 year to machined and electropolished implants with different oxide thicknesses. J Mater Sci Mater Med. 1997. 8:721–729.22. Ducheyne P, Beight J, Cuckler J, Evans B, Radin S. Effect of calcium phosphate coating characteristics on early post-operative bone tissue ingrowth. Biomaterials. 1990. 11:531–540.23. Chae JC, Collier JP, Mayor MB, Surprenant VA, Dauphinais LA. Enhanced ingrowth of porous-coated CoCr implants plasma-sprayed with tricalcium phosphate. J Biomed Mater Res. 1992. 26:93–102.24. Schopper C, Moser D, Goriwoda W, Ziya-Ghazvini F, Spassova E, Lagogiannis G, Auterith A, Ewers R. The effect of three different calcium phosphate implant coatings on bone deposition and coating resorption: a long-term histological study in sheep. Clin Oral Implants Res. 2005. 16:357–368.25. Clemens JA, Klein CP, Sakkers RJ, Dhert WJ, de Groot K, Rozing PM. Healing of gaps around calcium phosphate-coated implants in trabecular bone of the goat. J Biomed Mater Res. 1997. 36:55–64.26. Lee TM, Wang BC, Yang YC, Chang E, Yang CY. Comparison of plasma-sprayed hydroxyapatite coatings and hydroxyapatite/tricalcium phosphate composite coatings: in vivo study. J Biomed Mater Res. 2001. 55:360–367.27. Geesink RS, Groot KD, Klein CP. Bonding of bone to apatite-coated implants. J Bone Joint Surg Br. 1988. 70:17–22.28. Sennerby L, Thomsen P, Ericson LE. Early tissue response to titanium implants inserted in rabbit cortical bone. part 1. Light microscopic observations. J Mater Sci Mater Med. 1993. 4:240–250.29. Sennerby L, Thomsen P, Ericson LE. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992. 7:62–71.30. Biesbrock AR, Edgerton M. Evaluation of the clinical predictability of hydroxyapatite-coated endosseous dental implants: a review of the literature. Int J Oral Maxillofac Implants. 1995. 10:712–720.31. Schliephake H, Scharnweber D, Roesseler S, Dard M, Sewing A, Aref A. Biomimetic calcium phosphate composite coating of dental implants. Int J Oral Maxillofac Implants. 2006. 21:738–746.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the removal torque and a histomorphometric evaluation of the RBM treated implants with the RBM followed by laser treated implants: an experimental study in rabbits
- The effect of different surface treatment on the osseointegration and stability of implants
- Histomorphometric and Removal Torque Values Comparision of Rough Surface Titanium Implants
- A HISTOMORPHOMETRIC STUDY OF TWO DIFFERENT THREADED CP TITANIUM IMPLANTS
- Bone response of three different surface implants: histomorphometric and resonance frequency analysis in dogs