Endocrinol Metab.
2013 Sep;28(3):192-198. 10.3803/EnM.2013.28.3.192.
The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
- Affiliations
-
- 1Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. swchomd@gmail.com
- 3Department of Internal Medicine, National Medical Center, Seoul, Korea.
- 4Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 5Hansung Science High School, Seoul, Korea.
- 6Biomedical Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 8Department of Surgery, National Medical Center, Seoul, Korea.
- KMID: 1973823
- DOI: http://doi.org/10.3803/EnM.2013.28.3.192
Abstract
- BACKGROUND
Tumor-associated macrophages (TAMs) play a tumorigenic role related to advanced staging and poor prognosis in many human cancers including thyroid cancers. Yet, a functional role of TAMs in papillary thyroid carcinoma (PTC) has not been established. The aim of this study was to investigate TAM expression in human PTC with lymph node (LN) metastasis.
METHODS
Thirty-six patients who underwent surgery after being diagnosed with PTC with LN metastasis were included. Primary tumor tissues were immunohistochemically stained with an anti-CD68 antibody and clinical characteristics according to TAM density were evaluated.
RESULTS
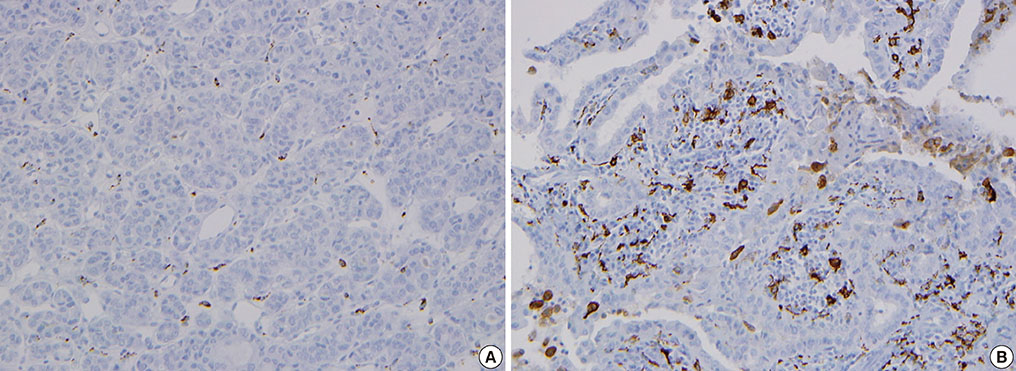
The TAM densities (CD68+ cells) varied from 5% to 70%, in all tumor areas, while few cells were stained in adjacent normal tissues. TAMs were identified as CD68+ cells with thin, elongated cytoplasmic extensions that formed a canopy structure over tumor cells. Comparing clinicopathologic characteristics between tumors with low (<25%) and high (25% to 70%) TAM densities, primary tumors were larger in the high density group than in the low density group (2.0+/-0.1 vs. 1.5+/-0.1; P=0.009).
CONCLUSION
TAMs were identified in primary PTC tumors with LN metastasis and higher TAM densities were related to larger tumor sizes, suggesting a tumorigenic role of TAMs in human PTCs.
MeSH Terms
Figure
Cited by 3 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
Shujun Sun, Xinliang Pan, Limin Zhao, Jianming Zhou, Hongzeng Wang, Yonghong Sun
Clin Exp Otorhinolaryngol. 2016;9(3):270-277. doi: 10.21053/ceo.2015.01305.Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
Jinsun Lim, Han Sai Lee, Jin-Hyung Heo, Young Shin Song
Endocrinol Metab. 2024;39(2):324-333. doi: 10.3803/EnM.2023.1885.
Reference
-
1. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3:23–35.2. Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003; 73:209–212.3. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002; 23:549–555.4. Ladusch M, Schaffner H, Ullmann L, Littmann M, Reimann S, Gindl P, Ambrosius H. Pre- and postoperative reactivity of breast cancer patients to tumor associated antigens and HEP in the macrophage electrophoresis mobility (MEM) test. Arch Geschwulstforsch. 1982; 52:469–478.5. Neumeister B, Hambsch K, Storch H. Macrophage adherence inhibition test (MAI) in Wistar rats bearing Jensen tumors. I. MAI after incubation with tumor-associated antigens. Arch Geschwulstforsch. 1983; 53:521–528.6. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002; 196:254–265.7. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011; 9:216.8. Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005; 14:425–431.9. Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, Lin A, Adeyanju OO, Li S, Gong C, McGrath M, Olopade OI, Esserman LJ. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011; 128:703–711.10. Hirayama S, Ishii G, Nagai K, Ono S, Kojima M, Yamauchi C, Aokage K, Hishida T, Yoshida J, Suzuki K, Ochiai A. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. J Thorac Oncol. 2012; 7:1790–1797.11. Sato S, Hanibuchi M, Kuramoto T, Yamamori N, Goto H, Ogawa H, Mitsuhashi A, Van TT, Kakiuchi S, Akiyama S, Nishioka Y, Sone S. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis. 2013; 30:333–344.12. Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7:e50946.13. Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, Aozasa K, Okuyama A, Nonomura N. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol. 2009; 181:1894–1900.14. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008; 15:1069–1074.15. Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, Chen X, Wang WQ, Li XY, Xiao JC, Ning G. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012; 22:905–910.16. Fiumara A, Belfiore A, Russo G, Salomone E, Santonocito GM, Ippolito O, Vigneri R, Gangemi P. In situ evidence of neoplastic cell phagocytosis by macrophages in papillary thyroid cancer. J Clin Endocrinol Metab. 1997; 82:1615–1620.17. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011; 11:750–761.18. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006; 66:605–612.19. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.20. Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M, Dupuy C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011; 6:e22567.21. Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004; 101:4560–4565.22. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992; 176:287–292.23. Penton-Rol G, Cota M, Polentarutti N, Luini W, Bernasconi S, Borsatti A, Sica A, LaRosa GJ, Sozzani S, Poli G, Mantovani A. Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J Immunol. 1999; 163:3524–3529.24. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003; 19:71–82.25. Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS One. 2013; 8:e54302.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- Calcification and Expression of Bone Morphogenetic Protein-4 in Papillary Thyroid Carcinoma
- Medullary and Papillary Thyroid Carcinoma as a Collision Tumor: Report of Five Cases
- Warthin-like Tumor Variant of Papillary Thyroid Carcinoma: A Case Report