Korean Circ J.
2015 Jul;45(4):325-332. 10.4070/kcj.2015.45.4.325.
Absence of Correlation between Changes in the Number of Endothelial Progenitor Cell Subsets
- Affiliations
-
- 1Department of Cardiovascular Medicine, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. aghasadk@sums.ac.ir
- 2Cell and Molecular Medicine Research Club, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 3Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 4Cardiovascular Research Center, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 5Department of OB-GYN, Division of Infertility and Reproductive Medicine, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 6Infertility and Reproductive Medicine Research Center, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 7Perinatology Research Center, Infertility Reaserch Center, Department of OB-GYN, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- 8Department of Immunology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 1964105
- DOI: http://doi.org/10.4070/kcj.2015.45.4.325
Abstract
- BACKGROUND AND OBJECTIVES
Previously, various methodologies were used to enumerate the endothelial progenitor cells (EPCs). We now know that these methodologies enumerate at least three different EPC subsets: circulating angiogenic cells (CACs), colony-forming unit endothelial cells (CFU-ECs), and endothelial colony-forming cells (ECFCs). It is not clear whether there is a correlation between changes in the number of these subsets. The aim of the current study is to find an answer to this question.
MATERIALS AND METHODS
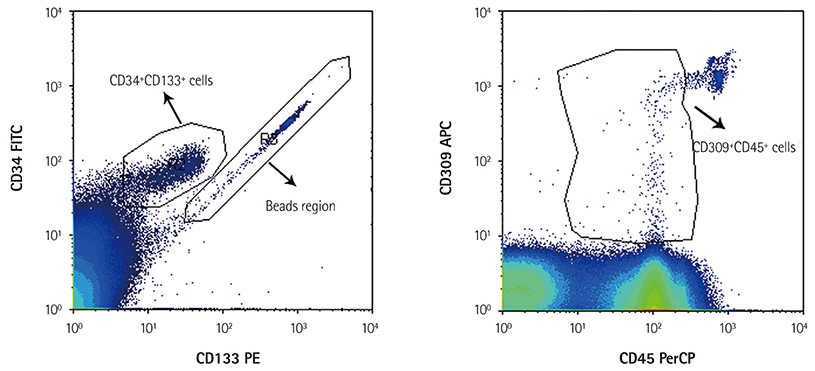
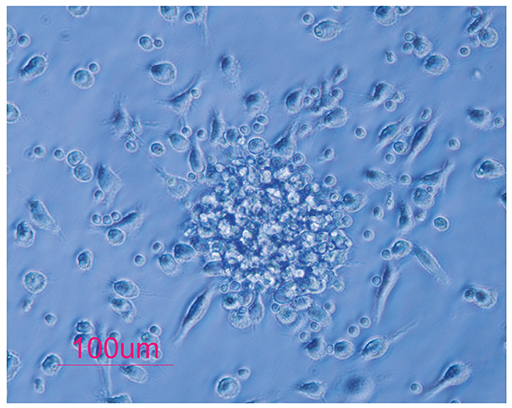
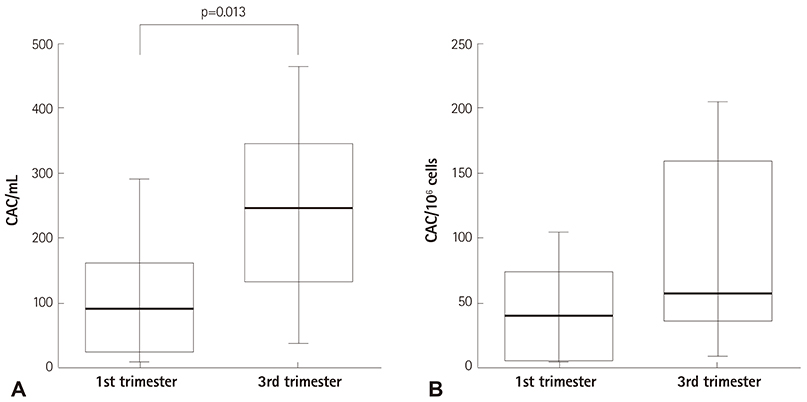
The number of all EPC subsets was quantified in the peripheral blood of nine pregnant women in their first and third trimesters of pregnancy. We enumerated 14 cell populations by quantitative flow-cytometry using various combinations of the markers, CD34, CD133, CD309, and CD45, to cover most of the reported phenotypes of CACs and ECFCs. Culturing technique was used to enumerate the CFU-ECs. Changes in the number of cells were calculated by subtracting the number of cells in the first trimester peripheral blood from the number of cells in the third trimester peripheral blood, and correlations between these changes were analyzed.
RESULTS
The number of CFU-ECs did not correlate with the number of ECFCs and CACs. Also, CACs and ECFCs showed independent behaviors. However, the number of CACs showed a strong correlation with the number of CD133+CD309+ cells (p=0.001) and a moderate correlation with the number of CD34+CD309+ cells (p=0.042). Also, the number of ECFCs was correlated with the number of CD309+CD45- cells (p=0.029) and CD34+CD45- cells (p=0.03).
CONCLUSION
Our study showed that the three commonly used methods for quantifying EPC subsets represent different cells with independent behaviors. Also, any study that measured the number of EPCs using the flow cytometry method with a marker combination that lacks CD309 may be inaccurate.
Keyword
MeSH Terms
Figure
Reference
-
1. Attar A, Khosravi Maharlooi M, Khoshkhou S, et al. Colony forming unit endothelial cells do not exhibit telomerase alternative splicing variants and activity. Iran Biomed J. 2013; 17:146–151.2. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967.3. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600.4. Lin C, Rajakumar A, Plymire DA, Verma V, Markovic N, Hubel CA. Maternal endothelial progenitor colony-forming units with macrophage characteristics are reduced in preeclampsia. Am J Hypertens. 2009; 22:1014–1019.5. Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000; 95:952–958.6. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005; 353:999–1007.7. Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009; 13:87–102.8. Güven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006; 48:1579–1587.9. Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007; 25:60–66.10. Schömig K, Busch G, Steppich B, et al. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006; 27:1032–1037.11. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008; 28:1584–1595.12. Ingram DA, Mead LE, Tanaka H, et al. Identifi cation of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004; 104:2752–2760.13. Povsic TJ, Zavodni KL, Vainorius E, Kherani JF, Goldschmidt-Clermont PJ, Peterson ED. Common endothelial progenitor cell assays identify discrete endothelial progenitor cell populations. Am Heart J. 2009; 157:335–344.14. Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources. J Transl Med. 2007; 5:37.15. George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006; 12:331–335.16. Parsanezhad ME, Attar A, Namavar-Jahromi B, et al. Changes in endothelial progenitor cell subsets in normal pregnancy compared with preeclampsia. J Chin Med Assoc. 2015; 78:345–352.17. Khan SS, Solomon MA, McCoy JP Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005; 64:1–8.18. Buemi M, Allegra A, D'Anna R, et al. Concentration of circulating endothelial progenitor cells (EPC) in normal pregnancy and in pregnant women with diabetes and hypertension. Am J Obstet Gynecol. 2007; 196:68.e1–68.e6.19. Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006; 98:e20–e25.20. Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005; 45:1441–1448.21. Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005; 25:296–301.22. Massa M, Campanelli R, Bonetti E, Ferrario M, Marinoni B, Rosti V. Rapid and large increase of the frequency of circulating endothelial colony-forming cells (ECFCs) generating late outgrowth endothelial cells in patients with acute myocardial infarction. Exp Hematol. 2009; 37:8–9.23. Davani S, Gozalo C, Gambert S, et al. The polymorphism Trp719Arg in the kinesin-like protein 6 is associated with the presence of late outgrowth endothelial progenitor cells in acute myocardial infarction. Atherosclerosis. 2010; 210:48–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regular Exercise Training Increases the Number of Endothelial Progenitor Cells and Decreases Homocysteine Levels in Healthy Peripheral Blood
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Endothelial Progenitor Cells' Classification and Application in Neurological Diseases
- Can improvements in our physiological understanding yield information on the utility of endothelial progenitor cell capture stents?
- Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration