J Korean Assoc Oral Maxillofac Surg.
2014 Feb;40(1):3-10. 10.5125/jkaoms.2014.40.1.3.
Controlled release of nerve growth factor from heparin-conjugated fibrin gel within the nerve growth factor-delivering implant
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Korea University Guro Hospital, Seoul, Korea.
- 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Seoul National University, Seoul, Korea. leejongh@snu.ac.kr
- KMID: 1960965
- DOI: http://doi.org/10.5125/jkaoms.2014.40.1.3
Abstract
OBJECTIVES
Although nerve growth factor (NGF) could promote the functional regeneration of an injured peripheral nerve, it is very difficult for NGF to sustain the therapeutic dose in the defect due to its short half-life. In this study, we loaded the NGF-bound heparin-conjugated fibrin (HCF) gel in the NGF-delivering implants and analyzed the time-dependent release of NGF and its bioactivity to evaluate the clinical effectiveness.
MATERIALS AND METHODS
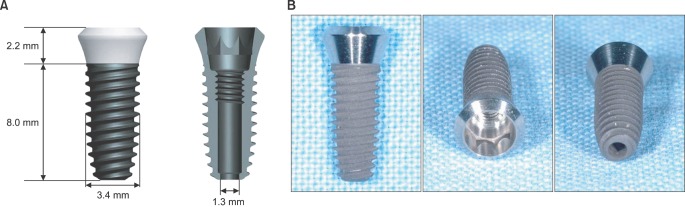
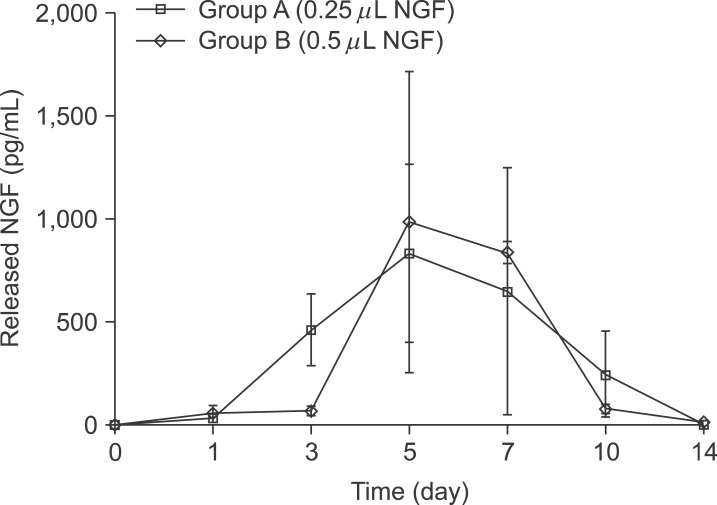
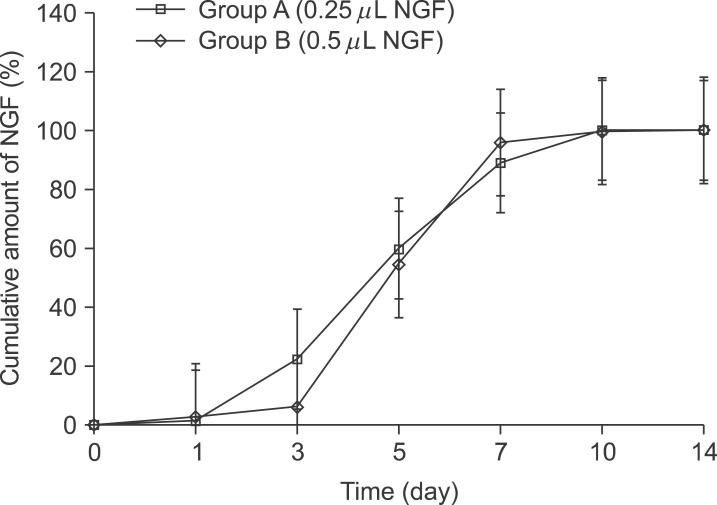
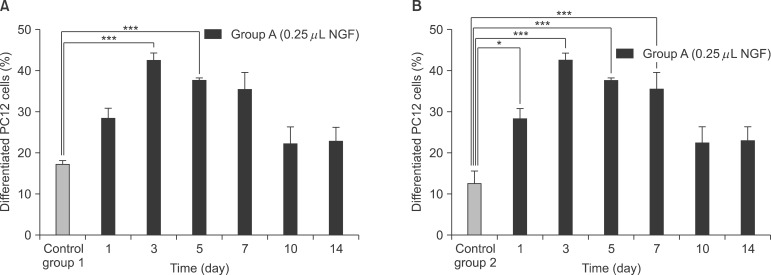
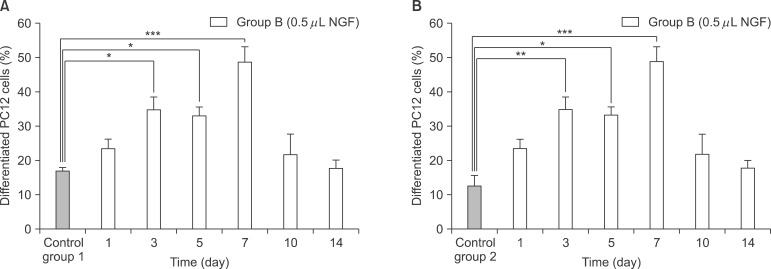
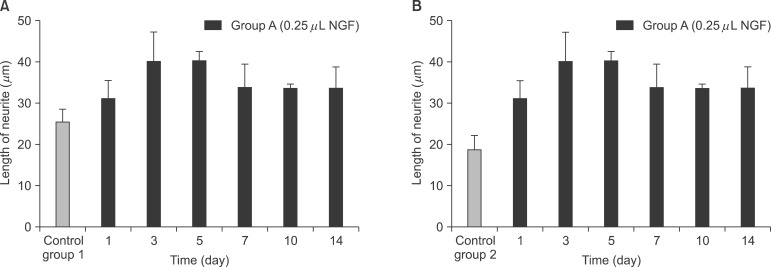
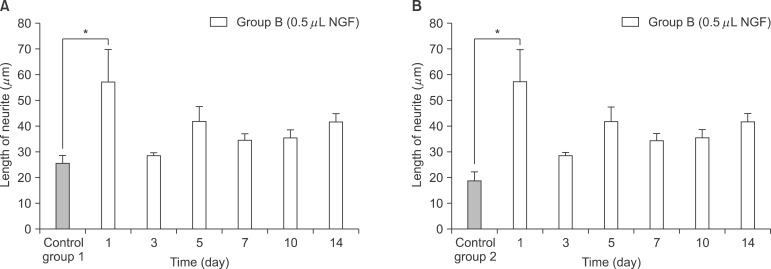
NGF solution was made of 1.0 mg of NGF and 1.0 mL of phosphate buffered saline (PBS). Experimental group A consisted of three implants, in which 0.25 microL of NGF solution, 0.75 microL of HCF, 1.0 microL of fibrinogen and 2.0 microL of thrombin was injected via apex hole with micropipette and gelated, were put into the centrifuge tube. Three implants of experimental group B were prepared with the mixture of 0.5 microL of NGF solution, 0.5 microL HCF, 1.0 microL of fibrinogen and 2.0 microL of thrombin. These six centrifuge tubes were filled with 1.0 mL of PBS and stirred in the water-filled beaker at 50 rpm. At 1, 3, 5, 7, 10, and 14 days, 1.0 mL of solution in each tubes was collected and preserved at -20degrees C with adding same amount of fresh PBS. Enzyme-linked immunosorbent assay (ELISA) was done to determine in vitro release profile of NGF and its bioactivity was evaluated with neural differentiation of pheochromocytoma (PC12) cells.
RESULTS
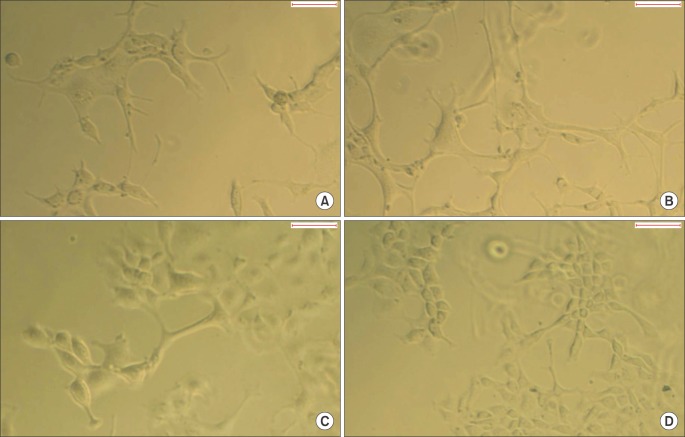
The average concentration of released NGF in the group A and B increased for the first 5 days and then gradually decreased. Almost all of NGF was released during 10 days. Released NGF from two groups could promote neural differentiation and neurite outgrowth of PC12 cells and these bioactivity was maintained over 14 days.
CONCLUSION
Controlled release system using NGF-HCF gel via NGF-delivering implant could be an another vehicle of delivering NGF to promote the nerve regeneration of dental implant related nerve damage.
Keyword
MeSH Terms
Figure
Reference
-
1. Renton T, Yilmaz Z. Profiling of patients presenting with post-traumatic neuropathy of the trigeminal nerve. J Orofac Pain. 2011; 25:333–344. PMID: 22247929.2. van Steenberghe D, Lekholm U, Bolender C, Folmer T, Henry P, Herrmann I, et al. Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: a prospective multicenter study on 558 fixtures. Int J Oral Maxillofac Implants. 1990; 5:272–281. PMID: 2098330.3. Kiyak HA, Beach BH, Worthington P, Taylor T, Bolender C, Evans J. Psychological impact of osseointegrated dental implants. Int J Oral Maxillofac Implants. 1990; 5:61–69. PMID: 2202671.4. Ellies LG, Hawker PB. The prevalence of altered sensation associated with implant surgery. Int J Oral Maxillofac Implants. 1993; 8:674–679. PMID: 8181830.5. Bartling R, Freeman K, Kraut RA. The incidence of altered sensation of the mental nerve after mandibular implant placement. J Oral Maxillofac Surg. 1999; 57:1408–1412. PMID: 10596660.
Article6. Tay AB, Zuniga JR. Clinical characteristics of trigeminal nerve injury referrals to a university centre. Int J Oral Maxillofac Surg. 2007; 36:922–927. PMID: 17875382.
Article7. Gordon T. The physiology of neural injury and regeneration: the role of neurotrophic factors. J Commun Disord. 2010; 43:265–273. PMID: 20451212.
Article8. Wood MD, Sakiyama-Elbert SE. Release rate controls biological activity of nerve growth factor released from fibrin matrices containing affinity-based delivery systems. J Biomed Mater Res A. 2008; 84:300–312. PMID: 17607752.
Article9. Magill CK, Moore AM, Yan Y, Tong AY, MacEwan MR, Yee A, et al. The differential effects of pathway- versus target-derived glial cell line-derived neurotrophic factor on peripheral nerve regeneration. J Neurosurg. 2010; 113:102–109. PMID: 19943736.
Article10. Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951; 116:321–361. PMID: 14824426.
Article11. Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994; 77:627–638. PMID: 8205613.
Article12. Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997; 18:1201–1225. PMID: 9300556.
Article13. Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol. 1996; 148:1807–1818. PMID: 8669468.14. Madduri S, Papaloïzos M, Gander B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials. 2010; 31:2323–2334. PMID: 20004018.
Article15. Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules. 2009; 10:174–183. PMID: 19061335.
Article16. Bhang SH, Jeon O, Choi CY, Kwon YH, Kim BS. Controlled release of nerve growth factor from fibrin gel. J Biomed Mater Res A. 2007; 80:998–1002. PMID: 17117469.
Article17. Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000; 69:149–158. PMID: 11018553.
Article18. Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010; 16:1225–1233. PMID: 19886733.
Article19. Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005; 105:249–259. PMID: 16088988.20. Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000; 65:389–402. PMID: 10699297.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma
- The study of nerve regeneration with infiltration of normal saline and nerve growth factor after vein graft to the resected sciatic nerve
- The study of nerve regeneration with infiltration of normal saline and nerve growth factor after vein graft to the resected sciatic nerve
- An Experimental Study of the Effect of Acidic Fibroblast Growth Factor on Nerve Regeneration
- Long-term Angiogenesis Efficacy Using a Heparin-Conjugated Fibrin (HCF) Delivery System with HBM-MSCs